More Information
Submitted: 18 September 2020 | Approved: 02 October 2020 | Published: 05 October 2020
How to cite this article: Cappelli A, Laureti S, Capozzi N, Mosconi C, Modestino F, et al. Percutaneous abdomino-pelvic abscess drainage in complicated Crohn’s disease. Ann Clin Gastroenterol Hepatol. 2020; 4: 045-051.
DOI: 10.29328/journal.acgh.1001022
ORCiD: orcid.org/0000-0003-1142-3917
Copyright License: © 2020 Cappelli A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Abdomino-pelvic abscesses; Percutaneous drainage; Crohn’s disease; Postoperative abscesses
Abbreviations: CD: Crohn’s Disease; PAD: Percutaneous Abscess Drainage; CS: Clinical Success; CF: Clinical Failure; TS: Technical Success; TF: Technical Failure; OS: Overall Success; OF: Overall Failure; OTS: Overall Technical Success; DS: Standard Deviation; p.o.: post-operative; CT: Computed Tomography; US: Ultrasonographic
Percutaneous abdomino-pelvic abscess drainage in complicated Crohn’s disease
Alberta Cappelli1*, Silvio Laureti2, Nunzia Capozzi1, Cristina Mosconi1, Francesco Modestino1, Giuliano Peta1, Silvia Lo Monaco1, Antonio Bruno1, Giulio Vara1, Caterina De Benedittis1, Paolo Gionchetti1, Fernando Rizzello1, Gilberto Poggioli1 and Rita Golfieri1
1Department of Radiology, IRCCS University Hospital of Bologna, via Albertoni 15, Bologna, Italy
2Department of Medical and Surgical Sciences, IRCCS University Hospital of Bologna, via Massarenti 9, Bologna, Italy
*Address for Correspondence: Alberta Cappelli, Department of Radiology, IRCCS University Hospital of Bologna, via Albertoni 15, Bologna, Italy, Tel: +39.051.2142598; Email: [email protected]
Purpose: Percutaneous abscess drainage (PAD) is the first-line approach for abscess in Crohn’s disease (CD) since it procrastinates or avoids surgery especially in postoperative abscesses [within 30 days post-operative (p.o.)]. We retrospectively evaluated the effectiveness, complications and outcome after PAD in postoperative and spontaneous abscesses and factors influencing the outcomes.
Methods: We performed PAD in 91 abscesses, 45 (49,5%) postoperative and 46 (50,5%) spontaneous.
We defined the overall success (OS) as clinical (CS) and technical success (TS) when imaging documented the resolution of the abscess with no surgery within 30 days.
Conversely, patients without abscess at the time of surgery, were considered as TS but clinical failure (CF).
We also analyzed the overall failure (OF) defined as CF with or without technical failure (TF).
Overall technical success (OTS) was OS plus TS. Complications were classified as major and minor according to the Interventional Radiology Criteria.
Results: In postoperative abscesses we found 91% OS, 9% OF, no TF and 100% OTS.
In spontaneous abscesses we found 33% OS, 67% OF, 6.4% TF, 95,6% OTS.
A total abscess resolution was achieved in 97,8% of patients. No major complication occurred; only 1 case of minor complication. Factors statistically influencing the outcome were postoperative vs spontaneous collections (OF: 9% vs. 67%, p < 0.0001), multiloculated vs uniloculated collections (OF: 38% vs. 1%, p < 0.0001) and upper abdominal vs lower location (OF: 13% vs. 25%, p <0.05).
Conclusion: Our data confirms the safety and effectiveness of PAD even in cases needing surgery within 30 days; most remarkable, PAD allows avoidance of early reoperation in almost all the patients with postoperative abscess.
Crohn’s disease (CD) is a chronic, relapsing inflammatory disorder of the gastrointestinal tract characterized by a transmural inflammation of the bowel wall leading to complications including perforation, fistula and abdominopelvic abscess onset. This latter event is present in a percentage of CD patients ranging between 20% - 30% [1-6].
In the past, patients with an abdominopelvic abscess were treated with surgical drainage followed by bowel resection. Currently, percutaneous abscess drainage (PAD) is considered the standard therapy for abdominopelvic infected fluid collections due to its significant reduction of morbidity and mortality compared to surgical procedures [1,7-14].
ECCO-ESCP guidelines published in 2018 state that intra-abdominal abscess should initially be treated with antibiotics and/or percutaneous drainage [15]. The safety and efficacy of PAD as first-line therapy has been demonstrated in several retrospective studies. In particular, studies reported high efficacy of PAD in postoperative collections in CD while spontaneous abscesses are more resistant to PAD due to their frequent association to fistulas and the underlying stenosis [4,7,9,16,17].
Nowadays, PAD has been employed with the goals of avoiding or better, delaying surgery until sepsis and clinical patients’ conditions are resolved or improved.
Technical approach and clinical success rates on patients’ series were widely reported in the literature [18] however, studies on the timing to surgery and factors affecting the clinical success rate are limited [19,20].
We retrospectively evaluated both the efficacy in terms of technical and clinical success rate, complications rate and final outcome of PAD in patients with abscesses from CD considering separately postoperative and spontaneous abscesses.
We also analyzed the factors which can potentially influence the efficacy of the procedure.
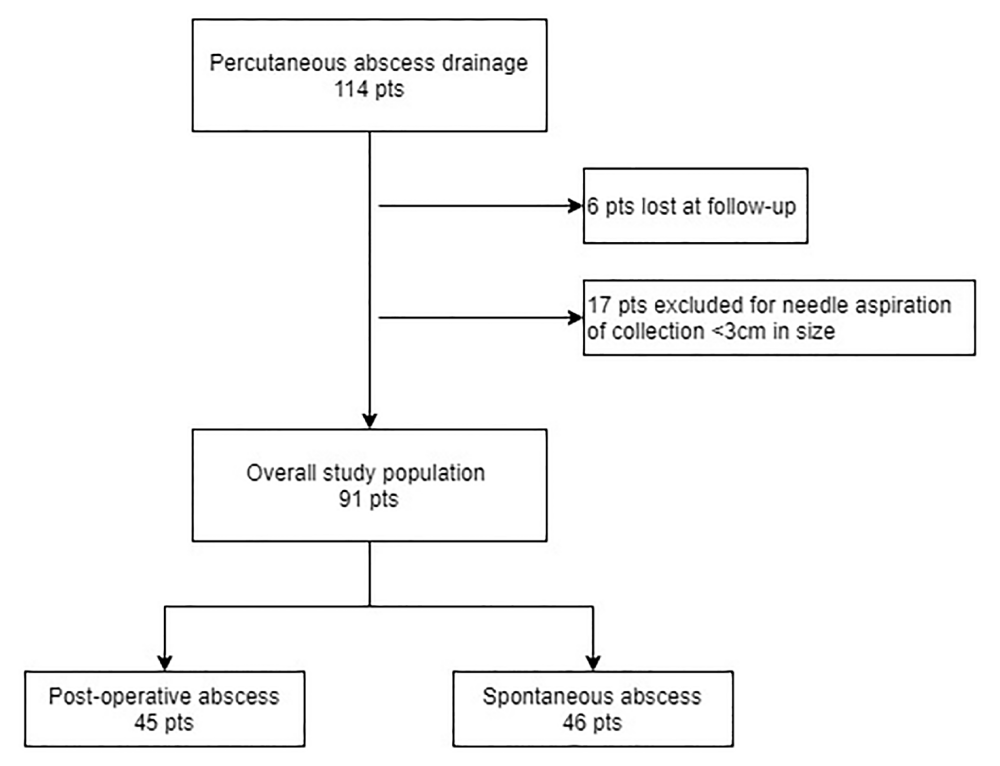
The present study fulfilled the Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data. The study conforms to the ethics guidelines of the Declaration of Helsinki and data collection and analyses had already previously been approved by Institutional Review Board of our hospital. Informed consent was obtained from patients who participated in clinical investigations. In the period between September 2006 and March 2019 at our Department we performed 114 PAD in patients with abscesses from CD. Six patients were excluded because lost at follow-up; in addition, we excluded 17 patients submitted to simple needle aspiration of fluid collection < 3 cm in diameter; therefore, our final study population resulted in 91 patients including 45 postoperative abscesses [occurred within 30 days post-operative (p.o.)] and 46 spontaneous “disease-related” abscesses (Figure 1).
Figure 1: Study population.
The inclusion criteria were fluid infected collections > 3 cm in diameter located in upper (right and/or left hypochondrium) or lower abdomen/pelvis (pelvis; right and/or left iliac fossa) associated to confirmed diagnosis of CD and signs or symptoms of sepsis (fever, leukocytosis, local pain). Postoperative collections were considered any abscess >3 cm occurring within 30 days from the abdominal surgery and documented at imaging.
All relevant clinical information (demographics, disease location, previous medical treatments, surgical history) were collected from our institutional database.
Short and long-term follow-up information (extended to at least 3 years in all patients) to verify the clinical outcome after PAD were collected.
For our purpose we defined the “Overall success” (OS) of the PAD as “Technical success” (TS) (complete resolution of the abscess documented by imaging) plus “Clinical success” (CS) (no need for surgery within the first 30 days post-drainage due to defervescence, decreased white blood cell count and improvement of the clinical condition of the patient).
Patients who needed surgery within 30 days after the procedure but had no residual abscess at the time of surgery were considered as TS but clinical failure (CF).
Technical failure (TF) was defined as the presence of a residual abscess. Overall Failure (OF) was defined as CF with or without TF.
Complications of PAD were classified as major or minor according to Interventional Radiology Criteria [21].
Any coagulopathy (INR > 1.5, platelet count < 50.000/mm3) was corrected and all the procedures were performed by an experienced interventional radiologist with at least 10 years of experience.
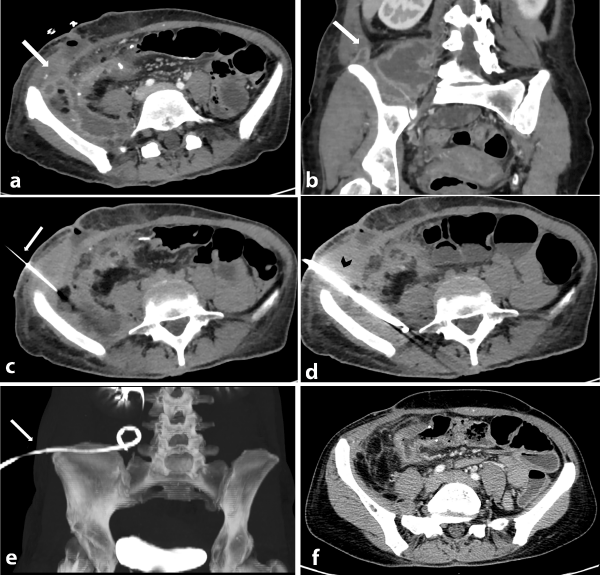
All catheters (8-10F; Flexima™ Boston Scientific Corporation) were placed with Seldinger technique via transabdominal or transgluteal routes according to the guidelines and appropriateness criteria reported in the literature [18,22,23] (Figure 2a-f).
Figure 2: Axial (a) and coronal (b) CT images show a post-operative multiloculated abscess in the right hypochondrium (white arrow) close to the ileocolic anastomosis; images show the involvement of the iliopsoas muscle and fluid around the collection. Axial (c) image shows the percutaneous abscess drainage (PAD) performed with Seldinger technique via transabdominal lateral approach due to the presence of bowel loops on the anterior side of the collection. Image shows the Chiba needle (white arrow) inserted into the collection preliminary to the insertion of the catheter. Axial (d) CT scan performed after the catheter positioning (black arrow head) shows the correct positioning of the catheter inside the wide component of the multiloculated abscess, confirmed by the coronal maximum intensity projection (MIP) CT image (white arrow in e). Axial (f) CT image performed 7 days after the PAD shows the complete resolution of the abscess without need of re-surgery for the patient.
After the placement of the catheter, systemic antibiotic therapy was given until the signs and symptoms of the infection abated (reduction of fever and leukocytosis) and catheters were kept patent by irrigation with 10-20 ml saline solution two to four times a day, depending on the viscosity of the fluid drained.
Patients were evaluated for catheter-related complications and/or fistulous communication with ultrasonographic (US; Esaote), computed tomography (CT; GE Healtcare Lighspeed VCT) or trans-drainage fistulography CT weekly based on clinical and radiological feature.
All catheters have been removed when an amount of daily drainage less than 10 mL/24 h was observed, and when providing it with a CT scan that confirmed a complete abscess resolution.
After the removal of the catheter, patients were followed with MR enterography (MR; GE Signa 1.5 T) yearly to evaluate both the evolution of the disease and the presence of fistulas and the relapse of the abscess.
Statistical analysis
All the analyses were performed using SPSS vs. 20 software. Categorical variables were expressed as frequencies and percentages, while the continuous ones as means and ranges. The significance of the differences between groups for continuous variables was assessed with the Student t test, whereas the chi square test of Pearson was applied for percentages. Significance was set at p < 0.05.
Main demographic and clinical characteristics of the study population are shown in table 1. The mean age of the 91 patients was 39 years (range 15-65 years) and 53 male and 38 female.
| Table 1: Demographic and clinical characteristics of the study population. | |
| N° patients | 91 |
| Male n° (%) Female n° (%) |
53 (58) 38 (42) |
| Mean age at the time of PAD, (range in years) | 39.7 (15-65) |
| Disease duration at the time of PAD (range in years) | 12.6 (0-41) |
| No previous Crohn’s-related surgery, n° (%) | 30 (33) |
| Spontaneous collection n°(%) Post-operative collection (within 30 days p.o.) n° (%) |
46 (51) 45 (49) |
| Recent/ongoing pharmacological therapy: steroids, n° (%) aminosalicylates, n° (%) immunosuppressants, n° (%) biologics, n° (%) |
43 (47) 24 (26) 5 (6) 11 (12) |
| Mean diameter of abscess in cm (range) | 6.5 (3-30) |
| Characteristics of the abscess Multiloculated abscess n° (%) Uniloculated abscess n° (%) |
20 (22) 71 (78) |
| Multiloculated post-operative n° (%) Multiloculated spontaneous n° (%) |
2 (4) 18 (39) |
| Documented fistula n° (%) | 67 (74) |
| Documented Fistula in spontaneous n° (%) | 35 (76) |
| Documented Fistula in post-operative n° (%) | 32 (71) |
| Location of largest abscess in the upper abdomen n° (%) Right hypochondrium Left hypochondrium |
33 (36) 11 (12) |
| Location of largest abscess in the lower abdomen n° (%) Pelvis Right iliac fossa Left iliac fossa |
17 (19) 27 (30) 3 (3) |
| Imaging modality assisting PAD n° (%) CT US |
86 (94) 5 (6) |
| Abbrevations: n°: number; PAD: Percutaneous abscess drainage; p.o.: Post-operative; CT: computed tomography; US: ultrasonographic | |
Most of the patients had a uniloculated abscess (71/91, 78%); among the 20/91 (22%) patients with a multiloculated abscess, the majority of them (18/20 cases) belong to the spontaneous group vs. 2/20 patients to the postoperative group.
We documented fistula tracts in 67 patients (74%) with similar distributions in postoperative and spontaneous abscesses (71% vs. 76% respectively).
Forty-four (44/91, 48%) abscesses were located in the upper abdomen divided in the right hypochondrium (33 patients, 36%), in the left hypochondrium (11 patients, 12%). On the opposite side, 47/91 (52%) abscesses resulted in the lower abdomen divided in the pelvis (17 patients, 19%), in the right iliac fossa (27 patients, 30%) and in the left iliac fossa (3 patients, 3%).
The mean diameter of the abscesses was 6.5 cm, ranging from 3 to 30 cm.
PAD was performed under CT guidance in 86 (94%) patients and US guidance in the remaining 5 (6%) cases. Drainage placement was performed without any major complications (septic shock, bacteremia, profuse bleeding requiring transfusion or bowel perforation) in all 91 patients. Only 1 patient experienced a minor complication (haematoma of the abdominal wall) resolved conservatively.
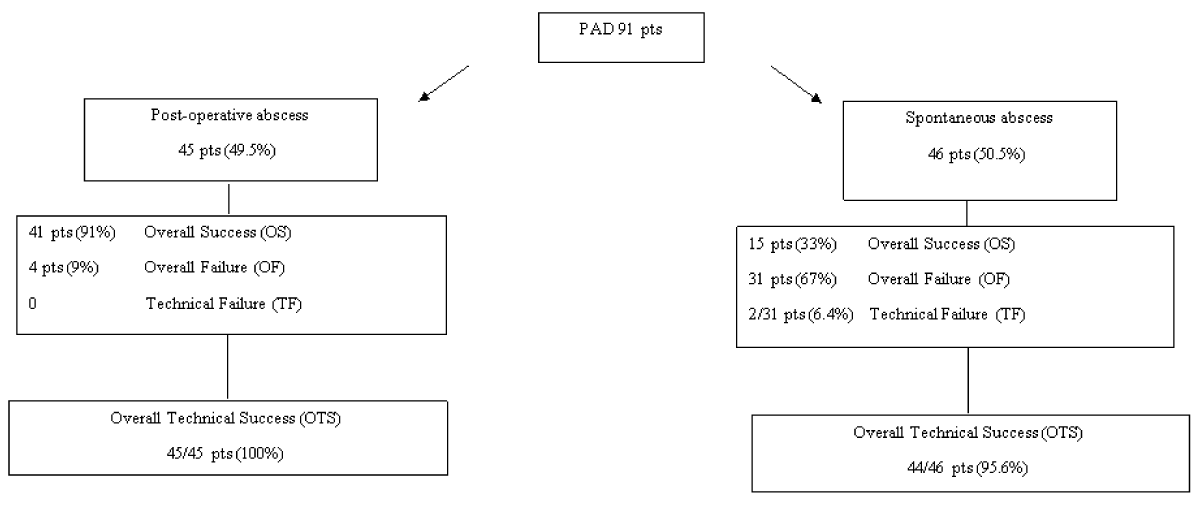
In the postoperative group we had OS 41 (91%), OF 4 (9%) and no TF; the global rate of technical success was 100%.
In the spontaneous group we had OS 15 (33%), OF 31 (67%) and TF 2 (6.4%); the global rate of technical success was 44 (95,6%).
Therefore, a total abscess drainage with resolution of the sepsis was achieved in the 89 (97,8%) of the whole study population.
The outcome of PAD is summarized in figure 3 and table 2.
Figure 3: Outcome after PAD - Overall success (OS): clinical (CS) and technical success (TS) = complete resolution of the abscess, with no need of surgery within 30 days - Technical Success (TS) but Clinical failure (CF) = complete resolution of the abscess at the time of surgery within 30 days after PAD - Technical Failure (TF): presence, total or partial, of the abscess at time of surgery within 30 days after PAD - Overall failure (OF): CF with or without TF - Overall technical success (OTS): OS plus TS.
We also analyzed which factors were potentially correlated with the outcome of the procedure (Table 2). Postoperative abscesses have been successfully resolved more frequently than spontaneous ones (p < 0.0001). We found no statistically significant differences among sex, age, abscess size and disease duration.
| Table 2: Outcome after PAD. | ||
| n° (%) | p | |
| Tot OS OF |
56 (62%) 35 (38%) |
p < 0.00001 |
| Post-operative OS OF Spontaneous OS OF |
41 (91%) 4 (9%) 15 (33%) 31 (67%) |
p < 0.00001 |
| Mean duration of drainage days (range) Post-operative Spontaneous |
15 (7-120) 11 21 |
p < 0.00001 |
| Mean length of hospitalization days (range) | 26 (1-116) | |
| Sex Male OS OF Female OS OF |
29 24 27 11 |
0.0086 |
| Size of collection 3-6 cm OS OF >7 cm OS OF |
30 20 26 15 |
0.9071 |
| Age median (DS) OS OF |
40 (12.3%) 41.5 (12.4%) |
0.585 |
| Age of disease median (DS) OS OF |
12.9 (10.1%) 13.5 (8.7%) |
0.751 |
| Location Upper abdomen OS OF Lower abdomen OS OF |
32 (36%) 12 (13%) 24 (26%) 23 (25%) |
< 0.05 |
| Multiloculation OS OF Uniloculation OS OF |
23 (26%) 35 (38%) 32 (35%) 1 (1%) |
p < 0.00001 |
| Abbrevations: OS: Overall Success; OF: Overall Failure | ||
Thirty-two (35.2%) of OS and 12 (13.2%) failures were achieved in the upper abdomen while 24 (26.4%) successes ad 23 (25.2%) failures in the lower abdomen/pelvis; these results to be statistically significant (p < 0.05) for a best outcome of upper location.
The characteristics of the abscess (multiloculated . uniloculated) were found to be statistically significant related to a predictor of success after PAD.
The mean length of hospitalization was 26 days (range 1-116 days) and the duration of drainage was of 15 days (range 7-120 days).
Interestingly, most of all the postoperative collections did not need early surgery; on the contrary, patients with a spontaneous abscess had an extremely variable clinical picture, regardless of the effectiveness of the drainage. Bowel resection was necessary within 1 month mainly due to obstructive symptoms, the coexistence of multiple fistulas, the presence of associated mesenterial phlegmons and poor quality of life in the patients. The substantial difference between the two subgroups was also evidenced by the discrepancy in the average length of hospitalization after drainage for the postoperative and spontaneous group (11 vs. 21 days respectively).
We also analyzed the influence of the pharmacological history of patients on the effectiveness of PAD.
Due to the difficulties in order to stratify patients on the basis of the therapies, we focused our attention on the use of biological drugs within 3 months from the onset of the abscess. Eleven out of 91 (12%) patients had biological drugs in their pharmacological history, consequently the sample’s meanness did not allow to draw meaningful conclusions.
Percutaneous drainage is an effective and safe procedure for CD complicated by an abdominopelvic abscess. The more recent ECCO-ESCP guidelines state that intra-abdominal abscess should initially be treated with antibiotics and/or percutaneous drainage and the surgical approach should be limited to the emergent cases [15]. Due to the extreme variability of the characteristics of collections patients and variability of CD itself, that can influence the success of the procedure, the curative rate of the drainage changes significantly between studies (38% - 100%) [8,24,25].
However, the efficacy of PAD is reported to be high for postoperative collections, while spontaneous abscesses are more resistant to PAD due to their frequent multiloculated features and the association with advanced post-inflammatory damages of the bowel wall mainly stenosis and fistulas [1,3,9,26-28].
Nowadays, it is well established that PAD is useful also in a preoperative setting in order to improve patients’ clinical conditions for further elective surgical procedure or better to avoid surgery.
However, its role in clinical management in severe complicated CD patients who need an early surgery, is not well established.
Data from different outcomes in postoperative and spontaneous abscesses which are different in the outcome due to the underlying clinical condition, are not available.
Our first goal was to retrospectively review the efficacy and safety of PAD both in postoperative and spontaneous abscess (this latter group included patients who needed an early surgery within 30 days).
Our main goal was to determine the rate of the OS of PAD defined as avoidance of surgery due to clinical improvement of the patients (CS) and to complete the resolution of the abscess (TS).
However, we added an additional criterion to better define the usefulness of PAD that is the clinical failure that means patients who needed surgery within 30 days from PAD due to the underlying severe disease but had a complete resolution of the abscess (TS).
This criterion was included because it is well known that PAD is used as a bridge to elective surgical bowel resection for severe persistent diseases complicated by strictures and fistulas.
In order to better discriminate our results, we considered data from postoperative and spontaneous abscesses separately.
Using these criteria, we found a global rate of technical success of 100% in the postoperative setting and 95.6% in the spontaneous group.
Our data is similar to those reported in the literature: in fact, it is well known that predictors of poor outcomes after PAD as a curative treatment are usually considered abscess characteristics (aetiology in terms of spontaneous or postoperative, unilocular or multilocular collections, location, size, number and presence of fistulas) [9,16,24,27,29-32].
We found a statistically significant correlation with a better outcome after PAD in patients with postoperative collections; one of the reasons may be the significant lower rate of multiloculated collections in this group that is 4% vs. 39% of the spontaneous group.
This latter characteristic of the abscess was related to a better outcome after PAD with a reported failure in 38% of the cases vs. 1% of the uniloculated ones (p < 0.0001).
Even though we found quite a similar number of documented fistulas in both Groups, the differences in fistula tract was evident particularly in the postoperative patients, fistulas were mainly due to an anastomotic dehiscence and the collection originated from a peritoneal contamination occurred at the time of surgery. On the other side in the spontaneous group fistulas are mainly related to severe intestinal stenosis which causes fistulas, deep intestinal wall ulcerations and poor clinical conditions; this condition makes it hard to achieve a high clinical success in this population. Akinci, et al. [20]. reported the presence of fistulas as the only factor affecting the clinical success rate after PAD in a group of 185 pelvic abscesses. Other authors reported that predictors of unsuccessful outcomes after PAD are abscesses caused by internal wide fistulas which is an indication for surgical repair in a range of 8%-18% of the cases [30,33,34-36].
Differently from other experiences in our study population, we did not find a strong difference in the OS on the basis of the collection location reporting a trend in the significance between success and failure in patients with a collection in the upper abdomen. A possible explanation could be the most complicated access routes through the abdominal wall, especially in postoperative patients; moreover, pelvic collections are often winding and supported by entero-vesical, entero-vaginal and entero-sigmoid fistulas (data non reported).
No statistically significant correlation was found between the outcome of the procedure and the assessed demographic variables such as gender, age of the patient at the time of hospitalization and previous years of illness.
Surprisingly, the size of the collection does not seem to influence significantly the success of the procedure either: both abscesses between 3-6 cm and those beyond 7 cm have shown similar outcomes.
As reported from other authors [1,27] we observed collections up to 13 cm resolved with a PAD. In our experience we did not find any major complications including septicemia with associated disseminated intravascular coagulation or hypotension, bowel perforation or death. Data from literature reports a complication rate both for spontaneous and postoperative abscesses in 8%-10% of the cases, mortality at 30 days ranging from 1% to 6% and puncture-related mortality in around 0.7% [33,34, 37]
This variability may be due to varying levels of expertise in interventional radiology and the management of Crohn’s patients in non-specialized centers.
Our better results may be referred to a preferential CT guided access technique due to the high number of deep collections.
The limitation of our paper is the retrospective evaluation, the limited number of patients and the absence of a stratification for side, severity and location of the disease.
In fact we know that an abscess that is successfully drained, but results in a fistula could not be considered a complete success of the drainage, but rather by delaying further surgery and allowing for patient optimization, therefore it is a success under a different light so we analyzed differently the technical and the clinical success.
In conclusion our data showed safety and effectiveness of PAD both in postoperative and spontaneous abscesses even in patients needing early surgery due to the severity of obstructive symptoms or active anastomotic fistulas.
- Xie Y, Zhu W, Li N, Li J. The outcome of initial percutaneous drainage versus surgical drainage for intra-abdominal abscesses in Crohn's disease. Int J Colorectal Dis. 2012; 27: 199-206. PubMed: https://pubmed.ncbi.nlm.nih.gov/22052039/
- de Groof EJ, Carbonnel F, Buskens CJ, Bemelman WA. Abdominal abscess in Crohn's disease: multidisciplinary management. Dig Dis. 2014; 32: 103-109. PubMed: https://pubmed.ncbi.nlm.nih.gov/25531361/
- Patil SA, Cross RK. Medical versus surgical management of penetrating Crohn's disease: the current situation and future perspectives. Expert Rev Gastroenterol Hepatol. 2017; 11: 843-848. PubMed: https://pubmed.ncbi.nlm.nih.gov/28633544/
- Bafford AC, Coakley B, Powers S, Greenwald D, Ha CY, Weintraub JM et al. The clinical impact of preoperative percutaneous drainage of abdominopelvic abscesses in patients with Crohn's disease. Int J Colorectal Dis. 2012; 27: 953-958. PubMed: https://pubmed.ncbi.nlm.nih.gov/22249438/
- Torres J, Mehandru S, Colombel JF, Peyrin-Biroulet L. Crohn’s disease. Lancet 2017; 389: 1741-1755. PubMed: https://pubmed.ncbi.nlm.nih.gov/27914655/
- Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn's disease. Dis Mon. 2018; 64: 20-57. PubMed: https://pubmed.ncbi.nlm.nih.gov/28826742/
- da Luz Moreira A, Stocchi L, Tan E, Tekkis PP, Fazio VW. Outcomes of Crohn's disease presenting with abdominopelvic abscess. Dis Colon Rectum. 2009; 52: 906-912. PubMed: https://pubmed.ncbi.nlm.nih.gov/19502855/
- Clancy C, Boland T, Deasy J, et al. A Meta-analysis of Percutaneous Drainage Versus Surgery as the Initial Treatment of Crohn’s Disease-related Intra-abdominal Abscess. J Crohns Colitis. 2016; 10: 202-208. PubMed: https://pubmed.ncbi.nlm.nih.gov/26512133/
- Golfieri R, Cappelli A, Giampalma E, Rizzello F, Gionchetti P, et al. CT-guided percutaneous pelvic abscess drainage in Crohn’s disease. Tech Coloproctol. 2006; 10: 99-105. PubMed: https://pubmed.ncbi.nlm.nih.gov/16773292/
- Pugmire BS, Gee MS, Kaplan JL, Hahn PF, Doody DP, et al. Role of percutaneous abscess drainage in the management of young patients with Crohn disease. Pediatr Radiol. 2016; 46: 653-659. PubMed: https://pubmed.ncbi.nlm.nih.gov/26833482/
- Hirten RP, Shah S, Sachar DB, Colombel JF. The Management of Intestinal Penetrating Crohn's Disease. Inflamm Bowel Dis. 2018; 24: 752-765. PubMed: https://pubmed.ncbi.nlm.nih.gov/29528400/
- Feagins LA, Holubar SD, Kane SV, Spechler SJ. Current strategies in the management of intra-abdominal abscesses in Crohn’s disease. Clin Gastroenterol Hepatol. 2011; 9: 842-850. PubMed: https://pubmed.ncbi.nlm.nih.gov/21679776/
- Gionchetti P, Dignass A, Danese S, Magro Dias FJ, Rogler G, et al. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 2: Surgical Management and Special Situations. J Crohns Colitis. 2017; 11: 135-149. PubMed: https://pubmed.ncbi.nlm.nih.gov/27660342/
- de Barros KSC, Flores C, Harlacher L, Francesconi CFM. Evolution of Clinical Behavior in Crohn's Disease: Factors Associated with Complicated Disease and Surgery. Dig Dis Sci. 2017; 62: 2481-2488. PubMed: https://pubmed.ncbi.nlm.nih.gov/28748409/
- Bemelman WA, Warusavitarne J, Sampietro GMM, et al. ECCO-ESCP Consensus on Surgery for Crohn's Disease. J Crohns Colitis. 2018; 12: 1-16. PubMed: https://pubmed.ncbi.nlm.nih.gov/28498901/
- Gervais DA, Hahn PF, O’Neill MJ, Mueller PR. Percutaneous abscess drainage in Crohn’s disease: technical success and short-term and long-term outcomes during 14 years. Radiology 2002; 222:645-651. PubMed: https://pubmed.ncbi.nlm.nih.gov/11867780/
- Cinat ME, Wilson SE, Din AM. Determinants for successful percutaneous image-guided drainage of intra-abdominal abscess. Arch Surg. 2002; 137: 845-849. PubMed: https://pubmed.ncbi.nlm.nih.gov/12093344/
- Ballard DH, Erickson AEM, Ahuja C, Vea R, Sangster GP, D'Agostino HB. Percutaneous management of enterocutaneous fistulae and abscess-fistula complexes. Dig Dis Interv. 2018; 2: 131-140. PubMed: https://pubmed.ncbi.nlm.nih.gov/31073548/
- Lobatón T, Guardiola J, Rodriguez-Moranta F, Millán-Scheiding M et al. Comparison of the long-term outcome of two therapeutic strategies for the management of abdominal abscess complicating Crohn's disease: percutaneous drainage or immediate surgical treatment. Colorectal Dis. 2013; 15: 1267-1272.
- Akinci D, Ergun O, Topel Ç, Çiftçi T, Akhan O. Pelvic abscess drainage: outcome with factors affecting the clinical success. Diagn Interv Radiol. 2018; 24: 146-152. PubMed: https://pubmed.ncbi.nlm.nih.gov/29770767/
- Dariushnia SR, Mitchell JW, Chaudry G, Hogan MJ. Society of Interventional Radiology Quality Improvement Standards for Image-Guided Percutaneous Drainage and Aspiration of Abscesses and Fluid Collections. J Vasc Interv Radiol. 2020; 31: 662-666.e4. PubMed: https://pubmed.ncbi.nlm.nih.gov/32061521/
- Patel IJ, Davidson JC, Nikolic B, Salazar GM, Schwartzberg MS, et al. Consensus guidelines for periprocedural management of coagulation status and hemostasis risk in percutaneous image-guided interventions. J Vasc Interv Radiol. 2012; 23: 727-736. PubMed: https://pubmed.ncbi.nlm.nih.gov/22513394/
- Harisinghani MG, Gervais DA, Hahn PF, Cho CH, Jhaveri K, et al. CT-guided transgluteal drainage of deep pelvic abscesses: indications, technique, procedure-related complications, and clinical outcome. Radiographics. 2002; 22: 1353-1367. PubMed: https://pubmed.ncbi.nlm.nih.gov/12432107/
- He X, Lin X, Lian L, Huang J, Yao Q, et al. Preoperative Percutaneous Drainage of Spontaneous Intra-Abdominal Abscess in Patients With Crohn's Disease: A Meta-Analysis. J Clin Gastroenterol. 2015; 49: e82-e90. PubMed: https://pubmed.ncbi.nlm.nih.gov/25216386/
- Lorenz JM, Al-Refaie WB, Cash BD, et al. ACR appropriateness criteria radiologic management of infected fluid collections. J Am Coll Radiol. 2015; 12: 791-799. PubMed: https://pubmed.ncbi.nlm.nih.gov/26145248/
- Yamaguchi A, Matsui T, Sakurai T, Ueki T, Nakabayashi S, et al. The clinical characteristics and outcome of intraabdominal abscess in Crohn's disease. J Gastroenterol. 2004; 39: 441-448. PubMed: https://pubmed.ncbi.nlm.nih.gov/15175942/
- Bermejo F, Garrido E, Chaparro M, Gordillo J, Mañosa M, et al.Efficacy of different therapeutic options for spontaneous abdominal abscesses in Crohn's disease: are antibiotics enough? Inflamm Bowel Dis. 2012; 18: 1509-1514. PubMed: https://pubmed.ncbi.nlm.nih.gov/22674826/
- Müller-Wille R, Iesalnieks I, Dornia C, Ott C, Jung EM, et al. Influence of percutaneous abscess drainage on severe postoperative septic complications in patients with Crohn's disease. Int J Colorectal Dis. 2011; 26: 769-774. PubMed: https://pubmed.ncbi.nlm.nih.gov/21286921/
- Sahai A, Belair M, Gianfelice D, Coté S, Gratton J, et al. Percutaneous drainage of intra-abdominal abscesses in Crohn’s disease: short and long-term outcome. Am J Gastroenterol, 1997; 92: 275-278. PubMed: https://pubmed.ncbi.nlm.nih.gov/9040205/
- Giangreco L, Di Palo S, Castrucci M, Angeli E, Staudacher C. Abdominal abscesses: their treatment and the study of prognostic factors. Minerva Chir. 1997; 52: 369-376. PubMed: https://pubmed.ncbi.nlm.nih.gov/9265119/
- Lambiase RE, Deyoe L, Cronan JJ, Dorfman GS. Percutaneous drainage of 335 consecutive abscesses.results of primary drainage with 1-year follow-up. Radiology. 1992; 184: 167-179. PubMed: https://pubmed.ncbi.nlm.nih.gov/1376932/
- Van Sonnenberg E, Wittich GR, Goodacre BW, et al. Percutaneous abscess drainage: update. World J Surg 2001; 25: 362-369. PubMed: https://pubmed.ncbi.nlm.nih.gov/11343195/
- Van Sonnenberg E, D’Agostino HB, Casola G et al. Percutaneous abscess drainage: current concepts. Radiology 1991; 181: 617-626. PubMed: https://pubmed.ncbi.nlm.nih.gov/1947068/
- Jaques P, Mauro M, Safrit H, Yankaskas B, Piggott B. CT features of intra-abdominal abscesses: prediction of successful percutaneous drainage. AJR Am J Roentgenol. 1986; 146: 1041-1045. PubMed: https://pubmed.ncbi.nlm.nih.gov/2421562/
- Gervais DA, Ho CH, O’Neill MJ, Arellano RS, Hahn PF, et al. Recurrent abdominal and pelvic abscesses: incidence, results of repeated percutaneous drainage, and underlying causes in 956 drainages. AJR Am J Roentgenol. 2004; 182: 463-466. PubMed: https://pubmed.ncbi.nlm.nih.gov/14736682/
- Yaari S, Benson A, Aviran E, Lev Cohain N, Oren R, Sosna J, et al. Factors associated with surgery in patients with intra-abdominal fistulizing Crohn's disease. World J Gastroenterol. 2016; 22: 10380-10387. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5175250/
- Mueller PR, vanSonnenberg E, Ferrucci JT Jr. Percutaneous drainage of 250 abdominal abscesses and fluid collections. Part II. Current procedural concepts. Radiology. 1984; 151: 343-347. PubMed: https://pubmed.ncbi.nlm.nih.gov/6709903/