More Information
Submitted: 07 November 2019 | Approved: 01 June 2020 | Published: 02 June 2020
How to cite this article: Wen YZ, Dai SJ, Cheng SF, Shi J, Ai JH. Transcatheter arterial chemoembolization combined with molecular targeted therapy for a patient with hepatocellular carcinoma with intrahepatic metastasis and main portal vein tumor thrombus: A case report and literature review. Ann Clin Gastroenterol Hepatol. 2020; 4: 036-038.
DOI: 10.29328/journal.acgh.1001020
Copyright License: © 2020 Wen YZ, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Hepatocellular carcinoma (HCC); Transcatheter arterial chemoembolization (TACE); Molecular targeted therapy; Individualized therapy
Transcatheter arterial chemoembolization combined with molecular targeted therapy for a patient with hepatocellular carcinoma with intrahepatic metastasis and main portal vein tumor thrombus: A case report and literature review
Yu Zhong Wen, Shi Jie Dai, Su Fen Cheng, Jun Shi and Jun Hua Ai*
Department of Hepatobiliary Surgery, The First Affiliated Hospital of Nanchang University, PR China
*Address for Correspondence: Jun Hua Ai, Department of Hepatobiliary Surgery, The First Affiliated Hospital of Nanchang University, Yongwaizheng Street No.17, Donghu District, Nanchang City, Jiangxi Province, PR China, Email: [email protected]
Hepatocellular carcinoma (HCC) is characterized by high morbidity, high recurrence, and high mortality rates. In China, the morbidity of HCC is fifth among all malignant tumors and HCC is the third most common cause of cancer-related deaths. Most HCC patients also have liver cirrhosis. Surgery is the sole curative method for HCC; however, many patients are diagnosed with HCC during its advanced stages so radical resection can no longer be performed. Therefore, the proportion of patients who undergo radical hepatectomy is less than 30%. Patients with mildly advanced HCC cannot undergo hepatectomy and thus transcatheter arterial chemoembolization (TACE) and/or biological targeted therapy are alternative options. However, data on the effects of TACE therapy or biological targeted therapy are limited. Therefore, an investigation of multimodal and individualized treatments is critical to ensure the best treatment. In June 2018, we treated an advanced HCC patient with multiple metastases and right portal vein tumor thrombus. The patient exhibited partial remission after undergoing treatment with TACE and crizotinib capsules for 1 month. The case and a literature review are reported here.
Hepatocellular carcinoma (HCC) is one the most common malignant tumors, with high rates of morbidity and mortality. Surgery is currently the only curative method for HCC. However, many patients are diagnosed with HCC at the advanced stage, and radical resection can no longer be performed. Patients with mildly advanced HCC cannot undergo hepatectomy, and thus transcatheter arterial chemoembolization (TACE) and/or biological targeted therapy are alternative options for these patients. In the present study, we report a patient who showed benefit from the combined treatment of TACE and crizotinib.
General clinical data
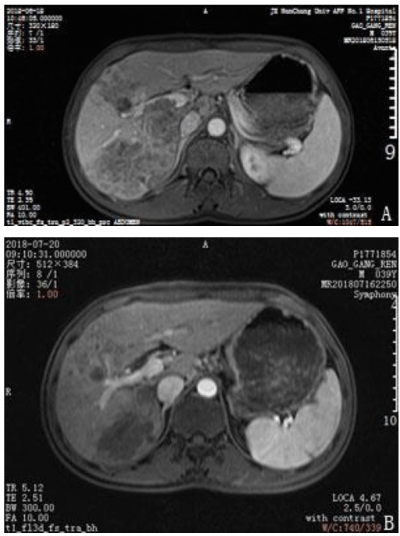
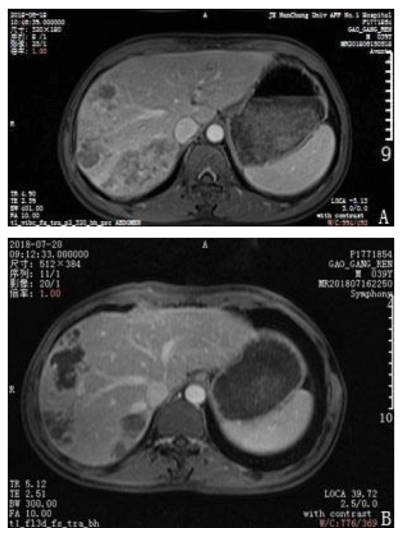
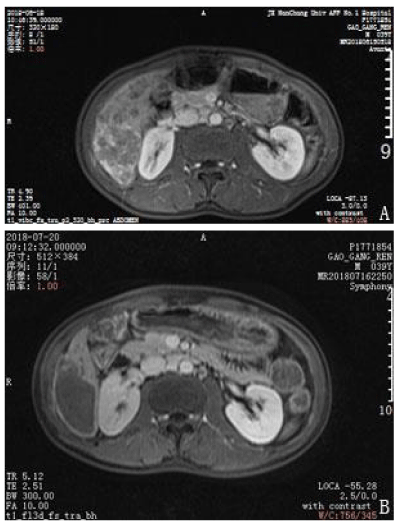
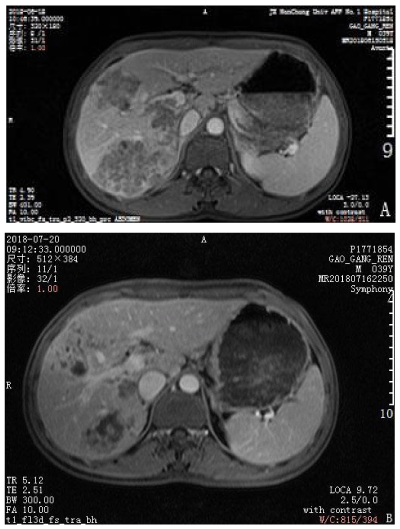
A 39-year-old man presented with upper abdominal pain that progressively intensified. The patient was diagnosed with gastric disease at a local hospital and received incorrect treatment. The patient’s urine was yellow and he lost 5 kg in body weight within 1 month. The patient had been positive for hepatitis s antigen, e antibody and c antibody for 10 years and had not accepted systemic anti-viral therapy. A physical examination was conducted and pain was evident in the upper abdomen. The liver was felt at 4 cm under the xiphoid appendix and was found to have a very hard texture and poor mobility. Furthermore, the patient was positive for hepatic percussion pain. Regarding the patient’s family history, his father had died from lymphadenoma. After hospitalization, the patient underwent magnetic resonance imaging (MRI) and an enhanced examination on 18 June 2018. Multiple intrahepatic lesions were identified and were determined as HCC with multiple intrahepatic metastases and right portal tumor thrombus (Figures 1–4A). Serum alpha-fetoprotein (AFP) levels were higher than 1210 ng/mL. No significant abnormal lesions were identified upon chest CT scan. On 20 June 2018, the patient underwent TACE. Eight milliliters of iodized oil, 40 mg perarubicin, and 50 mg lobaplatin were used during TACE. Gene sequencing revealed that the patient was sensitive to crizotinib, and the patient was administered oral crizotinib 250 mg twice a day. After treatment for 1 month, the patient returned to the hospital for reexamination on 20 July 2018 and underwent an MRI scan and enhanced examination. The results revealed multiple lesions in the liver and sedimented iodine in part of the lesions; the diameters of the lesions had significantly decreased compared with the previous lesions and the right portal vein tumor thrombus had disappeared. These results indicated that TACE and oral crizotinib treatment for 1 month caused significant shrinkage of the patient’s tumors (Figures 1–4B). The patient then stopped crizotinib treatment because he was short of funds. On 12 October 2018, the patient returned to the hospital for another reexamination. Serum AFP was 589.2 ng/mL and MRI and enhanced examination identified multiple intrahepatic lesions that had increased in number since the previous MRI. The patient died in February 2019.
Figure 1: Change of enhanced magnetic resonance imaging before treatment and after treatment. A. The right portal vein tumor thrombus before treatment with crizotinib. B. The right portal vein tumor thrombus disappeared after 1 month of crizotinib treatment.
Figure 2: Comparison of enhanced magnetic resonance imaging before treatment and after treatment. A. The right hepatic vein tumor thrombus before treatment with crizotinib. B. The right hepatic vein tumor thrombus had disappeared after 1 month of crizotinib treatment.
Figure 3: Comparison of enhanced magnetic resonance imaging before treatment and after treatment. A. Multiple tumors and bleeding in hepatic segment VI. B. The small tumors had disappeared and the center of the large tumor was necrotized in hepatic segment VI after 1 month of crizotinib treatment.
Figure 4: Comparison of enhanced magnetic resonance imaging before treatment and after treatment. A. The tumors were large before treatment. B. The tumor volume significantly decreased after 1 month of crizotinib treatment.
TACE is a common therapy for unresected HCC with portal vein tumor thrombosis and was the first treatment protocol for moderately advanced HCC [1]. Luo, et al. and Niu, et al. reported a survival rate of more than 1 year for TACE treatment and in a prospective study of TACE treatment for HCC, overall survival was longer in the TACE treatment group than the standard treatment group [2,3]. The blood supply for HCC is mainly from the hepatic artery, while 75% of the blood supply for normal liver tissues is from a portal vein. Thus, tumors that necrotize after chemotherapeutics are perfused into liver tumors via the hepatic artery, while the thromboembolic effects of lipiodol cause the chemotherapeutics to localize in the tumor region. The cytotoxicity of chemotherapeutics and the local ischemia from lipiodol result in synergistic necrosis of the liver tumor. However, killing all tumor cells is difficult if chemotherapeutics or lipiodol are used in isolation. Local ischemia and oxygen deficits activate specific vascular growth factors, which results in the angiogenesis of tumors, with new vessels and old vessels developing into a lateral branch and increasing the rate of recurrence and metastasis [4]. TACE combined with molecular targeted therapy is a new therapy for moderately advanced HCC and significantly decreases postoperative tumor recurrence [5]. In the present study, multiple intrahepatic metastases and right portal vein tumor thrombosis were identified in the patient. According to the diagnosis and treatment protocol of HCC in 2017, the patient had no indication for radical resection, and the best treatment for this patient was determined to be TACE combined with molecular targeted therapy.
At present, sorafenib and lenvatinib remain the only therapeutic drugs for moderately advanced HCC. Several clinical experimental studies have demonstrated that treatment with TACE combined with sorafenib could improve the prognosis of patients with HCC [6,7]. In the current patient, the copy number of MET, which encodes a tyrosine kinase receptor that is bound by hepatocyte growth factor as its ligand to activate multiple signaling pathways, was increased. According to the National Comprehensive Cancer Network (NCCN) protocol in 2018, crizotinib was authorized by the FDA for the treatment of non-small cell lung cancer with MET amplification and exon 14 skipping. The current patient was administered crizotinib for 1 month (250 mg, P.O, two times/day) after he underwent TACE. An MRI of the upper abdomen was then taken, and liver function and serum AFP were measured during a reexamination on 20 July 2018. The MRI showed that the diameter of the large tumor as well as the number of tumors had decreased, while serum AFP was significantly decreased, suggesting a very obvious therapeutic effect of TACE and crizotinib for this patient.
Sorafenib and lenvatinib remain the first-line therapies for moderately advanced HCC. However, individual differences in pathogenesis, mutagenic genes, recurrence, and metastasis are huge. Genetic testing revealed that the current patient was sensitive to crizotinib, and thus orally administered crizotinib treatment was recommended. While clinical benefits were observed in this patient, the treatment was halted because of insufficient personal funds.
In conclusion, individualized therapy is a promising therapeutic strategy for moderately advanced HCC.
We thank H. Nikki March, PhD, from Liwen Bianji, Edanz Editing China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
- Public Health and Family Planning Commission in the People's Republic of China. Specification for diagnosis and treatment of primary liver cancer. Chinese Journal of Practical Surgery. 2017; 37: 114-126.
- Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011; 18: 413–420. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20839057
- Niu ZJ, Ma YL, Kang P, Ou SQ, Meng ZB, et al. Transarterial chemoembolization compared with conservative treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus: using a new classification. Med Oncol. 2012; 29: 2992–2997. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22200992
- Sur BW, Sharma A. Transarterial Chemoembolization for Hepatocellular Carcinoma. J Radiol Nurs. 2018; 37: 107-111.
- Tian M, Zhang X, Huang G, Fan W, Li J, et al. Alpha-fetoprotein assessment for hepatocellular carcinoma after transarterial chemoembolization. Abdominal Radiology. 2019; 44: 3304-3311. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31250181
- Yuan J, Yin X, Tang B, Ma H, Zhang L, et al. Transarterial Chemoembolization(TACE) Combined with Sorafenib in Treatment of HBV Background Hepatocellular Carcinoma with Portal Vein Tumor Thrombus: A Propensity Score Matching Study. BioMed Res Int. 2019; 2019: 2141859. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6699376/
- Park JW, Kim YJ, Kim DY, Bae SH, Paik SW, et al. Sorafenib With or Without Concurrent Transarterial Chemoembolization in Patients With Advanced Hepatocellular Carcinoma: The Phase III STAH Trial. J Hepatol. 2019; 70: 684-691. PubMed: https://pubmed.ncbi.nlm.nih.gov/30529387/