More Information
Submitted: April 28, 2025 | Approved: May 31, 2025 | Published: June 02, 2025
How to cite this article: Ajao FO, Adeyeye IO, Kalejaiye NO, Mukaila SO, Agboola OS, Iyedupe MO. The Synergistic Effect of Combined Linagliptin and Metformin Improves Hepatic Function in High-fat Diet/Streptozotocin-induced Diabetic Rats. Ann Clin Gastroenterol Hepatol. 2025; 9(1): 004-012. Available from:
https://dx.doi.org/10.29328/journal.acgh.1001050.
DOI: 10.29328/journal.acgh.1001050
Copyright license: © 2025 Ajao FO, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Diabetes; Liver function; Dyslipidemia; Oxidative stress and Inflammation; Linagliptin; Metformin
The Synergistic Effect of Combined Linagliptin and Metformin Improves Hepatic Function in High-fat Diet/Streptozotocin-induced Diabetic Rats
Folasade Omobolanle Ajao1*, Ifedolapo Opeyemi Adeyeye1, Noheem Olaoluwa Kalejaiye1, Sodik Olasunkami Mukaila1, Olalekan Samson Agboola2 and Marcus Olaoye Iyedupe1
1Physiology Department, Faculty of Basic Medical Science, College of Health Science, Ladoke Akintola University of Technology, P.M.B. 4000, Ogbomoso, Oyo State, Nigeria
2Department of Veterinary Physiology and Biochemistry, University of Ibadan, Ibadan, Nigeria
*Address for Correspondence: Folasade Omobolanle Ajao, Department of Physiology, Ladoke Akintola University of Technology, P.M.B. 4000 Ogbomoso, 210214, Nigeria, Email: [email protected]
Background: Monotherapy for liver dysfunction in diabetes is less effective. This study investigated the effect of combined linagliptin and metformin therapy on liver function in diabetic rats.
Methods and materials: Sixty-four mature male (200-300 g) Wistar rats were used. Streptozotocin (35 mg/kgb.wt) was repeatedly injected intraperitoneally to induce diabetes. The rats were grouped into eight groups (n = 8). Group I: control; Group II: control + 10 mg/kgb.wt linagliptin; Group III: control + 200 mg/kgb.wt metformin; Group IV; control + 10 mg/kgb.wt linagliptin + 200 mg/kgb.wt metformin; Group V: diabetic; Group VI: diabetic + 10 mg/kgb.wt linagliptin; Group VII: diabetic + 200 mg/kgb.wt metformin; Group VIII: diabetic + 10 mg/kgb.wt linagliptin + 200 mg/kgb.wt metformin. The animals were sacrificed on the last day of the experiment, blood and liver samples were collected for biochemical assay.
Results: Insulin, blood glucose, glycated hemoglobin, total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-cholesterol), liver function biomarkers, liver glucose metabolic enzymes, malondialdehyde and inflammatory markers increased (p < 0.05) significantly. High-density lipoprotein-cholesterol (HDL-cholesterol), liver antioxidant, glycogen, and glycogen synthase were reduced significantly in diabetic rats. Linagliptin and metformin administration single and combined reduced the insulin, blood glucose, glycated hemoglobin, total cholesterol, triglycerides, LDL-cholesterol, liver function biomarkers, liver glucose metabolic enzymes, malondialdehyde, and inflammatory markers, and increased the HDL-cholesterol, liver antioxidant, glycogen and glycogen synthase in diabetic rats
Conclusion: Linagliptin monotherapy alone efficiently controls hyperglycemia and remarkably improves liver functions. Combining linagliptin and metformin could be used as safe and effective therapy for liver dysfunction progression in diabetes.
The global prevalence of diabetes mellitus is continually rising, and the data forecasted by the International Diabetes Federation estimated that approximately 783 million individuals will be living with diabetes by 2045 [1].
Diabetes Mellitus (DM) is a metabolic disease characterized by chronic hyperglycemia as a result of alterations in carbohydrate, protein, and fat metabolism due to absolute or relative insulin secretion from pancreatic beta-cells, insulin resistance, or a combination of both [2]. Persistent hyperglycemia causes micro-vascular and macro-vascular complications in diabetes, including neuropathy, nephropathy, retinopathy, cardiovascular diseases, arteriosclerosis, and liver diseases [3]. The overwhelming of endogenous antioxidant enzymes by free radicals via oxidative stress and inflammatory responses in tissues, which are critically implicated of organ dysfunctions related to diabetes [4,5]
The liver is a crucial organ for body glucose homeostasis regulation [6]. Non-alcoholic fatty liver disease (NAFLD), is a major hepatic disease that frequently develops in nearly 70% of type-2 diabetes patients, with a prevalence of 25% to 30% of the global population [7,8]. NAFLD is a multifactorial liver disease, characterized by excessive fat deposition in hepatocytes, independent of high alcohol intake, viral hepatitis, and drug-induced hepatotoxicity [9]. NAFLD can progress from mild to severe complications such as non-alcoholic steatohepatitis (NAHS), liver fibrosis, liver cirrhosis, and hepatocellular carcinoma [7,9,10]. Accumulation of lipid in the liver via consuming a high-calorie diet has been suggested to contribute significantly to the etiology of insulin resistance in the liver and NAFLD pathogenesis. This activates a series of oxidative and inflammatory responses that cause hepatic damage [11]. In an animal experimental model of NAFLD, a high-fat diet altered the hepatic histology and triggered the progression of metabolic abnormalities in the liver [12].
Metformin is the universal first-line drug choice for treating diabetes [13]. However, the ineffectiveness of metformin monotherapy in managing diabetes as the disease progresses necessitates a drug combination therapy for diabetes-related complications [14].
Linagliptin is one of the recent dipeptidyl peptidase-4 (DPP-4) inhibitors approved for the treatment of hyperglycemia [15,16]. Linagliptin has become a significant second and third-line oral anti-diabetic agent owing to the adverse effects and failure of metformin to optimally manage the progression of hyperglycemic conditions [17]. Studies have reported the anti-inflammatory, nephroprotective, and testicular injury amelioration of linagliptin in normal glycemic animals without inducing hypoglycemia conditions [18-20]. Also, the cardiovascular effect of linagliptin in adults with T2DM has been evaluated [21]. Linagliptin monotherapy or combined with metformin is well tolerated, produces better glycemic control and has currently been used to manage hyperglycemia in metformin-intolerant diabetic patients [22,23]. Previously, linaglptin and metformin combined therapy avert liver fibrosis in a newly diagnosed type-2 diabetes and NAFLD [24]. Nonetheless, the potential of linagliptin and metformin combined therapy on liver function abnormalities associated with NAFLD in diabetes still unexplored. This present research investigated the effect combination of linagliptin and metformin on hepatic function in high-fat diet and streptozotocin-induced diabetic rats.
Drugs and chemicals
Metformin, linagliptin, glucose, streptozotocin, ketamine, xylazine, phosphate buffer.
Experimental animals
A total of 64 mature male Wistar rats (200-300 g) were procured from the Animal House of Physiology Department, Ladoke Akintola University of Technology (LAUTECH), Ogbomoso, Oyo State, Nigeria. The animals were acclimatized for a week and kept in clean, hygienic polypropylene cages fed with a pelletized mash grower’s feed and water ad libitum under a standard pathogen-free environmental condition, temperature (25 23 °C ± 2 23 °C), relative humidity (45% ± 5%) and (12:12 hour’s) light/dark cycles. After the acclimatization period, the animals were fed with a high-fat diet (HFD) containing 45% (kcal %), 40% carbohydrate and 15% protein for 8 weeks. All experimental procedures were performed according to the protocol of the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and approved and assigned the ethical approval number ERCFBMSLAUTECH: 041/06/2024 by LAUTECH Faculty of Basic Medical Science Research Ethical committee.
Diabetes induction
The HFD was withdrawn from the animals, and allowed the animals to fast overnight (12 hours) prior to Streptozotocin (STZ) injection. After the fasting period, the rats were injected intraperitoneally with a single dose of freshly prepared STZ (35 mg/kg body weight) and given a 2% glucose solution to inhibit drug-induced hypoglycemic death. Diabetes induction was confirmed 72 hours post-STZ injection via tail vein blood sampling and glucometer analysis and the blood glucose level was taken with a one-touch Accu-chek glucometer. Animals with fasting blood glucose levels ≥ 200 mg/dL were considered diabetic and selected for the experiment.
Animal grouping
Diabetic rats and non-diabetic rats (control) were randomly assigned into 4 groups each, 8 rats/group treated with metformin (Met) and linagliptin (Lina) as detailed below:
Group I: Control (non-diabetic rats)
Group II: Control + 10 mg/kg body weight Lina
Group III: Control + 200 mg/kg body weight Met
Group IV: Control + 10 mg/kg body weight Lina + 200 mg/kg body weight Met
Group V: Diabetic (untreated)
Group VI: Diabetic + 10 mg/kg body weight Lina
Group VII: Diabetic + 200 mg/kg body weight Met
Group VIII: Diabetic + 10 mg/kg body weight Lina + 200 mg/kg body weight Met
The treatment phase lasted for 6 weeks. Food and water intake were recorded daily, while body weight and blood glucose were recorded weekly throughout the experiment.
Biochemical assay
The rats were fasted overnight (12 hours) and anesthetized with ketamine (40 mg/kg body weight) xylazine (20 mg/kg body weight) at the end of the experiment. The animals were sacrificed by cervical dislocation and fasting blood samples were collected from the apex beat of the rats’ hearts. The liver was isolated immediately after blood collection, rinsed in normal saline and homogenized in a freshly prepared cold phosphate-buffered. The blood samples were centrifuged at 3,500 rpm for 15 minutes at -4 °C and the liver tissues homogenates were centrifuged at 5000 rpm for 10 minutes at -4 °C. The clear supernatant serum obtained from the blood and plasma obtained from the liver homogenates after centrifugation were used to determine the biochemical parameters
Fasting blood glucose levels were determined using the glucose-oxidase/peroxidase (GOD-POD) method with a digital Accu-Chek glucometer and test strips via pricked tail vein blood.
Glycated hemoglobin (HbA1c) was estimated using a rat glycated hemoglobin assay kit (Fortress, UK) according to the manufacturer’s instructions.
Enzyme-linked immunosorbent assay (ELISA) was employed to determine the serum insulin, tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), using the specific parameters rat ELISA kit (Elabscience, China) followed the protocol of the manufacturer’s instructions.
Enzymatic colorimetric method was used to estimate serum total cholesterol (TC), triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C), with commercially available assay kits (Fortress, UK) following the manufacturer’s protocol. Friedewald et al. formula [25], was used to estimate the LDL-C as follows: LDL-c = TC - HDL - TG/5.
Liver alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels were measured by enzyme-linked immunosorbent assay (ELISA) using ALT and AST rat ELISA kit. Liver lactate dehydrogenase (LDH) and alkaline phosphatase (ALP) activity were assessed spectrophotometrically with available commercial kit (Fortress, UK). The enzymatic colorimetric method was used to determine liver glucose-6-phosphate dehydrogenase (G6PD) [26]. Liver glycogen and glycogen synthase were estimated based on the modified method of Tan et al. [27]. Liver pyruvate kinase was measured based on the Krishnan et al. [28] adopted method.
ELISA was used to assess the liver malondialdehyde (MDA), catalase (CAT), and reduced glutathione (GSH) activity via available commercial assay kits according to the manufacturer’s instructions, and glutathione disulfide (GSSG) was determined via the spectrophotometric technique.
Statistical analysis
Data were expressed as the standard error of the mean and analyzed with GraphPad Prism software (version 5.0) using one-way analysis of variance (ANOVA) followed by Bonferroni posthoc multiple test for group statistical significant comparison. Differences were considered statistically significant at p < 0.05.
Effect of combined linagliptin and metformin on body weight, liver weight, food intake, and water intake in HFD/STZ-induced diabetic rats
The body and liver weight of the diabetic rats significantly (p < 0.05) decreased, and the food and water intake increased significantly in comparison with the treated and non-treated control rats. Treatment with linagliptin and metformin, both singly and in combination, improved the body and liver weight and normalized the food and water intake compared with untreated diabetic rats (Table 1).
| Table 1: Effect of combined linagliptin and metformin on insulin, blood glucose, glycated hemoglobin, body weight, liver weight, food and water intake in high-fat diet/streptozotocin-induced diabetic rats - changes in metabolic parameters following treatment. | ||||||||
| Groups Parameters | Control | Control + 10 mg/kg body weight Lina |
Control + 200 mg/kg body weight Met | Control + 10 mg/kg body weight Lina + 200 mg/kg body weight Met | Diabetic control | Diabetic + 10 mg/kg body weight Lina | Diabetic+ 200 mg/kg body weight Met | Diabetic + 10 mg/kg body weight Lina + 200 mg/kg body weight Met |
| Serum Insulin (mIU/L) | 4.34 ± 0.05 | 5.33 ± 0.27 | 4.29 ± 0.22 | 4.50 ± 0.29 | 11.58 ± 0.69X | 4.97 ± 0.57T | 5.61 ± 0.48T | 5.53 ± 0.55G |
| Serum FBG (mg/dL) | 70.20 ± 1.69 | 72.00 ± 2.21 | 74.40 ± 3.04 | 76.20 ± 1.74 | 259.40 ± 3.57X | 121.00 ± 3.32T | 126.40 ± 5.30T | 108.09 ± 5.15G |
| Serum HbA1c (%) | 6.26 ± 0.29 | 7.15 ± 0.15 | 7.37 ± 0.17 | 7.29 ± 0.81 | 12.20 ± 0.36X | 7.93 ± 0.47T | 6.25 ± 0.58T | 7.67 ± 0.36G |
| Food intake (g/day/rat) | 29.80 ± 0.66 | 27.20 ± 0.86 | 29.80 ± 0.66 | 25.60 ± 1.69 | 39.40 ± 0.87X | 28.20 ± 0.80T | 31.80 ± 1.08T | 30.80 ± 1.20G |
| Water intake (ml/day/rat) | 38.20 ± 1.28 | 37.60 ± 2.25 | 35.00 ± 1.00 | 38.60 ± 1.17 | 58.60 ± 1.17X | 39.80 ± 1.56T | 38.20 ± 1.28T | 30.40 ± 1.17G |
| Body weight (g) | 303.60 ± 5.19 | 293.00 ± 2.09 | 297.40 ± 2.50 | 289.00 ± 3.06 | 190.60 ± 1.36X | 233.4 ± 3.95T | 255.40 ± 3.69T | 237.60 ± 2.50G |
| Liver weight (g) | 4.40 ± 0.26 | 4.10 ± 1.12 | 4.24 ± 0.23 | 4.50 ± 0.23 | 3.50 ± 0.10X | 3.86 ± 0.13T | 3.94 ± 0.09T | 4.36 ± 2.23G |
| The data values are presented as SEM (n = 8). Xsignificant at p < 0.05 vs. control; Tsignificant at p < 0.05 vs. diabetic group; Gsignificant at p < 0.05 vs. treated linagliptin and metformin-alone diabetic groups. | ||||||||
Effect of combined linagliptin and metformin on insulin, blood glucose and glycated hemoglobin in HFD/STZ-induced diabetic rats
There was a significant (p < 0.05) increase in insulin, blood glucose, and glycated hemoglobin (HbA1c) in diabetic rats compared with treated and non-treated control rats. The combined and single administration of linagliptin and metformin to the diabetic rats reduced the insulin, blood glucose and HbA1c levels compared with non-treated diabetic rats (Table 1).
Effect of combined linagliptin and metformin on liver function in HFD/STZ-induced diabetic rats
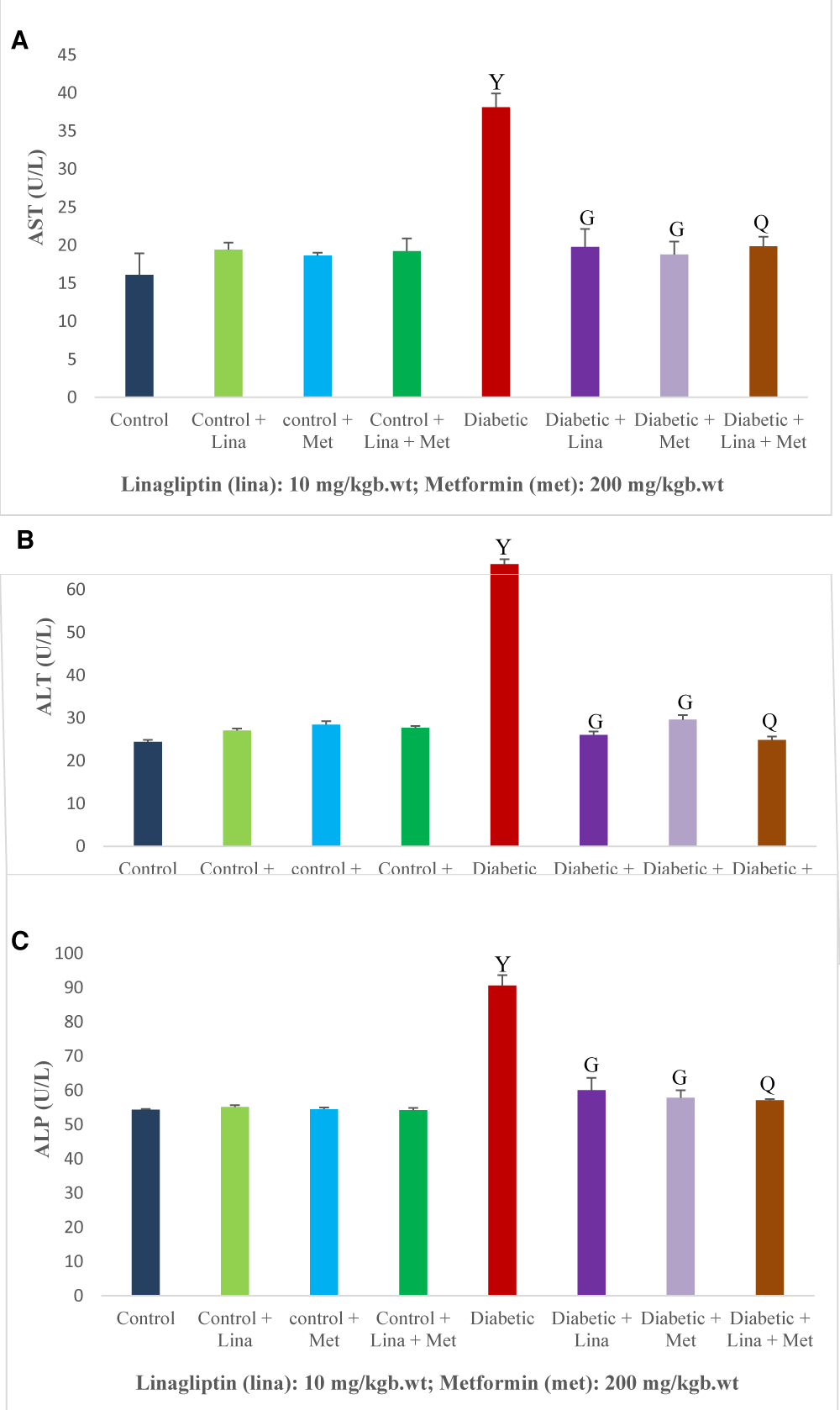
The diabetic rats had a significant (p < 0.05) increase in aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase compared with the treated and non-treated control rats. Administration of linagliptin and metformin in single and combined reduced the liver level of aspartate aminotransferase, alanine aminotransferase, and alkaline phosphatase in the diabetic rats compared with the non-treated diabetic rats (Figure 1 A-C).
Figure 1: Effect of combined linagliptin and metformin on liver enzymes in high-fat diet/streptozotocin-induced diabetic rats. (A) Aspartate aminotransferase (AST), (B) Alanine aminotransferase (ALT), and (C) Alkaline phosphatase (ALP). Treatment significantly reduced liver enzyme levels in diabetic rats, indicating hepatic protective effects of the combined therapy. Values are expressed as mean ± SEM (n = 8). Ysignificant at p < 0.05 compared with control; Gsignificant at p < 0.05 compared with untreated diabetic group; Qsignificant at p < 0.05 compared linagliptin and metformin-alone treated diabetic groups.
Effect of combined linagliptin and metformin on lipid profile in HFD/STZ-induced diabetic rats
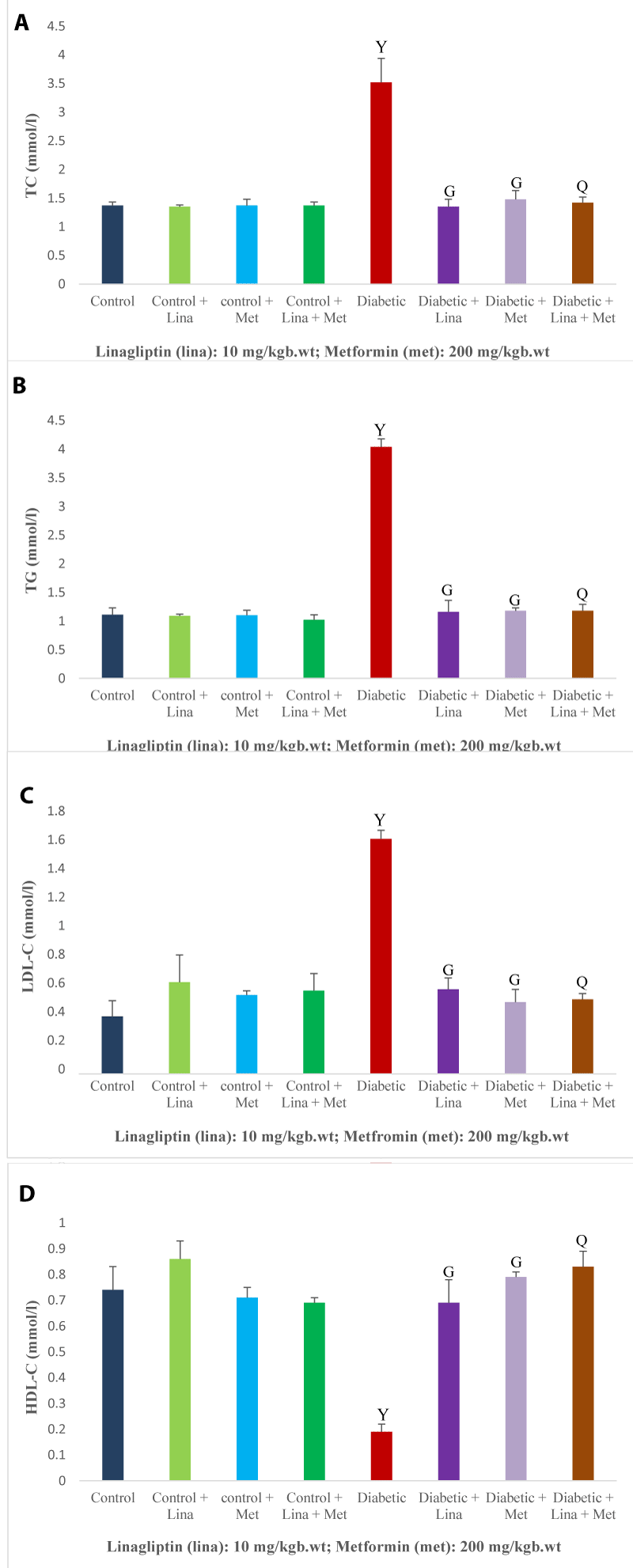
The levels of total cholesterol, triglycerides, and low-density lipoprotein cholesterol were significantly (p < 0.05) higher, and high-density lipoprotein cholesterol level was significantly lower in the diabetic rats compared with the treated and non-treated control rats. Linagliptin and metformin administration in single and combined to diabetic rats significantly lowered the total cholesterol, triglycerides, and low-density lipoprotein cholesterol levels, and increased the high-density lipoprotein cholesterol level in comparison with untreated diabetic rats (Figure 2 A-D).
Figure 2: Effect of combined linagliptin and metformin on lipid profile in high-fat diet/streptozotocin-induced diabetic rats. (A) Total cholesterol (TC), (B) Triglycerides (TG), (C) Low-density lipoprotein cholesterol (LDL-C), and (D) High-density lipoprotein cholesterol (HDL-C). Combined treatment improved lipid profile by lowering TC, TG, LDL-C and increasing HDL-C levels in diabetic rats. Values are expressed as mean ± SEM (n = 8). Ysignificant at p < 0.05 compared with control; Gsignificant at p < 0.05 compared with untreated diabetic group; Qsignificant at p < 0.05 compared linagliptin and metformin alone-treated diabetic groups.
Effect of combined linagliptin and metformin on liver oxidative stress and antioxidants enzymes in HFD/STZ-induced diabetic rats
There was a significant (p < 0.05) elevation in the liver oxidative stress marker malondialdehyde and a reduction in catalase, reduced glutathione (GSH), and glutathione disulfide (GSSG) in the diabetic rats compared in comparison with the treated and non-treated control rats. Diabetic rats treated with single and combined linagliptin and metformin had a significant reduction in liver malondialdehyde and an increase in catalase, GSH, and GSSG compared with the untreated diabetic rats (Table 2).
| Table 2: Effect of combined linagliptin and metformin on oxidative stress and inflammatory markers in high-fat diet/streptozotocin-induced diabetic rats — modulation of oxidative and inflammatory responses following treatment. | ||||||||
| Groups Parameters | Control | Control + 10 mg/kg body weight Lina | Control + 200 mg/kg body weight Met | Control + 10 mg/kg body weight Lina + 200 mg/kg body weight met | Diabetic control | Diabetic + 10 mg/kg body weight Lina | Diabetic + 200 mg/kg body weight Met | Diabetic + 10 mg/kg body weight Lina + 200 mg/kg body weight Met |
| Liver MDA (µM) | 1.17 ± 0.01 | 1.12 ± 0.04 | 0.87 ± 0.03 | 0.98 ± 0.07 | 2.04 ± 0.16* | 1.08 ± 0.04α | 1.17 ± 0.09α | 1.13 ± 0.09β |
| Liver CAT (u/mg protein) |
30.58 ± 0.75 | 30.23 ± 1.03 | 31.82 ± 0.95 | 28. 09 ± 1.39 | 14. 59 ± 0.49* | 29. 00 ± 1.73α | 28.68 ± 1.09α | 29.62 ± 2.33β |
| Liver GSH (mM) | 0.18 ± 0.01 | 0.19 ± 0.02 | 0.18 ± 0.01 | 0.18 ± 0.02 | 0.10 ± 0.02* | 0.19 ± 0.04α | 0.19 ± 0.02α | 0.20 ± 0.03β |
| Liver GSSG (mM) | 0.03 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.01 ± 0.00* | 0.04 ± 0.00α | 0.03 ± 0.00α | 0.03 ± 0.00β |
| Serum TNF-α (pg/ml) | 363.22 ± 3.66 | 371.42 ± 5.01 | 328.33 ± 5.79 | 386.56 ± 5.45 | 844.03 ± 4.78* | 328.31 ± 9.33α | 370.95 ± 3.29α | 383.01 ± 5.38β |
| Serum IL-1β (pg/ml) | 58.69 ± 4.94 | 45.76 ± 2.27 | 42.82 ± 1.92 | 45.67 ± 4.84 | 68.35 ± 3.52 | 46.04 ± 6.79α | 49.86 ± 0.89α | 46.95 ± 5.12β |
| Serum IL-6 (pg/ml) | 15.46 ± 0.58 | 16.99 ± 0.82 | 16.79 ± 0.91 | 16.73 ± 0.64 | 33.97 ± 0.81 | 15.66 ± 1.30α | 16.04 ± 0.70α | 15.58 ± 1.44β |
| Values are expressed as SEM (n = 8). *significant at p < 0.05 against normal control; αsignificant at p < 0.05 against with untreated diabetic group; βsignificant at p < 0.05 against linagliptin and metformin-alone treated diabetic groups. | ||||||||
Effect of combined linagliptin and metformin on inflammatory markers in HFD/STZ-induced diabetic rats
The levels of tumor-necrosis factor-alpha, interleukin-1β, and interleukin-6 significantly (p < 0.05) elevated in the diabetic rats compared with non-treated and treated control rats. The single and combined administration of linagliptin and metformin to the diabetic rats significantly decreased the tumor necrosis factor-alpha, interleukin-1β, and interleukin-6 compared to the non-treated diabetic rats (Table 2).
Effect of combined linagliptin and metformin on liver glycogen and glucose metabolism enzymes in HFD/STZ-induced diabetic rats
Liver glycogen and glycogen synthase reduced (p < 0.05) significantly and lactate dehydrogenase (LDH), glucose-6-phosphate dehydrogenase (G6PD) and pyruvate kinase increased significantly in diabetic rats in comparison with treated control and non-treated control. Administration of linagliptin and metformin singly and combined to the diabetic rats increased the liver glycogen and glycogen synthase and significantly reduced the LDH, G6PD, and pyruvate kinase compared with untreated diabetic rats (Table 3).
| Table 3: Effect of combined linagliptin and metformin on glycogen content and glucose metabolism enzymes in high-fat diet/streptozotocin-induced diabetic rats — improvement in hepatic glucose metabolism following treatment. | ||||||||
| Groups Parameters | Control | Control + 10 mg/kg body weight Lina |
Control + 200 mg/kg body weight Met |
Control + 10 mg/kg body weight Lina + 200 mg/kg body weight Met |
Diabetic control | Diabetic + 10 mg/kg body weight Lina |
Diabetic + 200 mg/kg body weight Met |
Diabetic + 10 mg/kg body weight Lina + 200 mg/kg body weight Met |
| Liver glycogen | 4.45 ± 1.21 | 5.73 ± 0.82 | 6.15 ± 0.30 | 5.11 ± 1.24 | 2.39 ± 0.28Z | 5.09 ± 1.02Y | 5.33 ± 1.33Y | 6.11 ± 0.14Q |
| Liver LDH (U/L) | 593.48 ± 23.98 | 412.62 ± 54.58 | 491.02 ± 42.26 | 518.78 ± 52.38 | 905.70 ± 18.31Z | 524.78 ± 31.01Y | 487.64 ± 17.09Y | 565.67 ±65.28Q |
| Liver G6PD (U/L) | 85.80 ± 44.05 | 68.37 ± 13.08 | 64.48 ± 20.11 | 88.39 ± 8.53 | 162.44 ± 7.86Z | 85.31 ± 7.21Y | 101.69 ± 52.47Y | 95.73 ± 5.85Q |
| Liver pyruvate kinase (mmol/l) | 0.83 ± 0.15 | 0.76 ± 0.18 | 0.87 ± 0.08 | 0.76 ± 0.16 | 1.54 ± 0.26Z | 0.87 ± 0.06Y | 0.94 ± 0.11Y | 0.86 ± 0.15Q |
| Liver glycogen synthase (um/mg protein) | 1.41 ± 0.31 | 1.63 ± 0.07 | 1.48 ± 0.32 | 1.49 ± 0.05 | 0.84 ± 0.04Z | 1.56 ± 0.17Y | 1.62 ± 0.26Y | 1.54 ± 0.21Q |
| Data are presented as mean ± SEM (n = 8). Zsignificant at p < 0.05 compared with control; Ysignificant at p < 0.05 compared with diabetic group; Qsignificant at p < 0.05 compared linagliptin and metformin alone diabetic groups. | ||||||||
Diabetes is a disease of metabolic abnormalities frequently marked by hyperglycemia that causes many pathological conditions over time. Studies have reported that Non-alcoholic fatty liver disease (NAFLD) is the most common liver complication associated with diabetes with a lack of novel therapy [29]. Despite the liver’s essential role in regulating glucose and lipid metabolism, liver therapy in diabetes is still challenging [6,30]. This study investigates the single and combined potential of linagliptin and metformin in improving liver function in diabetic rats.
Diabetes disrupts glucose utilization for energy, causing catabolism of structural proteins and muscle tissue which leads to body weight reduction in diabetes [31]. Consistent with the study of Kondalkar et al. [32], diabetic rats exhibited a reduction in body and liver weights accompanied by elevated insulin, blood glucose, polyphagia, and polydipsia in this study. An increase in insulin level observed is a compensatory mechanism to elevated blood glucose. Several diabetes drugs have been studied for anti-hyperglycemic effects via stimulating the glucose-utilizing organs to insulin action for glucose uptake, and inhibiting hepatic gluconeogenesis, and increasing hepatic glycogenesis [33,34]. Linagliptin and metformin alone and co-administration improve the body and liver weight of diabetic rats and restore the insulin and blood glucose to the normal circulating level. In harmony with the report of Lv et al. [23], the combined therapy demonstrated significant hypoglycemic efficacy and could be linked to the mechanism previously reported above.
Glycated hemoglobin (HbA1c), the standard and predictor biomarker for diagnosing and assessing glycemic control, has been elevated in diabetic patients [35]. The level of HbA1c increased in the diabetic rats of the present study, parallel with the Alatawi et al. findings [36]. The combination of linagliptin and metformin lowered the HbA1c in the rats, indicating the combined treatment efficiently regulated insulin action and facilitated target organ responsiveness to insulin thereby lowering blood glucose and subsequently reducing hemoglobin glycation for HbA1c production, which is also in harmony with Lv et al. [23] findings on normalizing of glycated hemoglobin with combined linagliptin and metformin.
Liver impairment is a critical complication associated with chronic hyperglycemia in diabetes [6]. Elevated liver enzymes including alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase are biomarkers of liver injury in diabetes [37]. This study observed liver damage in diabetic rats marked by an increase in alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase which is consistent with the findings of Attia et al. [38]. The increase in liver enzymes could result from the deficit in hepatic glucose utilization which stimulated the release of the enzymes from the liver [39]. The combined treatment with linagliptin and metformin effectively diminished the liver enzyme biomarkers superiorly to the effect observed in linagliptin and metformin monotherapy, implies the liver protective effect of the combined treatment which supports the findings of Li et al. [24] on attenuation of liver fibrosis in newly diagnosed T2DM patients with NAFLD treated with combination of linagliptin and metformin.
Lactate dehydrogenase is a key energy metabolism enzyme. In diabetic individuals, disruptions in the level of this enzyme have been reported. Increased lactate dehydrogenase activity associated with metabolic impairments, including altered glucose uptake and impaired lactate synthesis, consequently triggered diabetic-related complications [40], which is consistent with our findings, where liver lactate dehydrogenase levels were elevated in diabetic rats. Glucose-6-phosphate dehydrogenase (G6PDH) is an enzyme that catalyzes the pentose phosphate to reduced form Nicotinamide Adenosine Dinucleotide Phosphate (NADPH), which regulates redox reaction in many cells, and alteration in G6PDH activity can cause oxidative stress-induced tissue injury [41]. A decrease in liver G6PDH activity in diabetics which leads to elevated oxidative stress in the cells has been reported [42], contrary to our finding, the diabetic rats had elevated G6PDH in the liver. Pyruvate kinase is another important glycolysis enzyme that facilitates the breakdown of phosphoenolpyruvate to pyruvate with the production of ATP energy [43]. Many findings have documented a decline in pyruvate kinase activity in diabetic rats [44], which is contrary to the result of the present findings, the level of pyruvate kinase remarkably increased in the liver of diabetic rats. Glycogen synthase/phosphorylase are two major controlling enzymes crucial for glycogen synthesis and lysis [45]. Glycogen synthesis and formation are critically influenced by high levels of glycogen synthase and uncontrolled hyperglycemia causes a diminution in glycogen synthase levels in muscle and liver resulting in lower glycogen storage [46]. In line with the findings of Govindarajan et al. [47], this study observed a decrease in glycogen synthase in the diabetic liver, which may contribute to decreased hepatic glycogen storage. The abnormalities in the glucose metabolism enzymes and reduced liver glycogen may be attributed to impaired liver insulin insensitivity and glucose utilization. The combination of linagliptin and metformin reduces LDH, G6PDH, and pyruvate kinase levels and ameliorates liver glycogen synthase, suggesting the potential of the combined treatment to regulate glucose metabolic enzymes to increase hepatic glycogen storage via inhibition of excessive breakdown of glycogen into glucose which consequently lowered the elevated blood glucose and which is consistent with the report of Unger and Cherrington [48].
Lipid profile abnormalities known as dyslipidemia is usually characterized by elevated levels of circulating blood total cholesterol, triglycerides, low-density lipoprotein-cholesterol and reduced high-density lipoprotein-cholesterol are metabolic derangements linked to etiology of cardio-vascular disease in diabetes [49]. Non-alcoholic fatty liver disease in diabetes has been reported as a contributing factor to the increase in total cholesterol, triglycerides, low-density lipoprotein-cholesterol and reduced high-density lipoprotein-cholesterol [50]. In agreement with the Komorizono et al. [51] and Lin et al. [52] reports, linagliptin and metformin combination therapy had anti-dyslipidemic efficacy than single drug therapy by lowering the total cholesterol and low-density lipoprotein-cholesterol considerably and improving the high-density lipoprotein-cholesterol in diabetic rats of this study and this lipid-lowering effect of combined therapy could be attributed to the synergistic action on insulin to stimulate the metabolism of fatty acid and triglycerides to reduce hepatic lipid deposition.
Diabetes-induced oxidative stress causes overproduction of reactive oxygen species and a diminished antioxidant defense system is implicated in the progression of micro and macro-vascular complications in diabetic patients [53]. Research has documented that elevated circulating liver enzymes in diabetes are a consequence of free radicals overwhelming liver antioxidant capacity leading to tissue injury [54]. Malondialdehyde, which is the product of lipid peroxidation serves a marker of oxidative stress, dominating the activity of catalase, reduced glutathione, and reduced disulfide antioxidant in current diabetic rats’ liver, consistent with Zeng et al. finding [55]. The combination of linagliptin and metformin remarkably reverses the malondialdehyde and improves the antioxidant defense enzyme activity in the liver, demonstrating superior antioxidant efficacy of the combined treatment over monotherapy.
Chronic hyperglycemia-induced dyslipidemia-triggered oxidative stress production is considered a crucial process linked with the pathogenesis of the inflammatory response in diabetes. upregulation of tumor necrosis factor-alpha, interleukin-1β, and interleukin-6 in the blood contributes to the development of NAFLD via stimulating the inflammatory pathways that disrupt the insulin signal [56]. The serum concentration of pro-inflammatory cytokines tumor necrosis factor-alpha, interleukine-1β, and interleukine-6 in the diabetic rats of this study increased which is in line with the previous finding of Lodhi et al. [57]. Both the single and combined administration of linagliptin and metformin produced anti-inflammatory properties, this finding supports other reports [18]. Although linagliptin monotherapy most effectively down-regulates the tumor necrosis factor-alpha, interleukin-1β, and interleukin-6, while the combined therapy demonstrated minimal efficacy. This anti-inflammatory property could be linked to the suppression of hyperglycemia and oxidative stress processes in the liver.
Linagliptin monotherapy exhibited superior anti-hyperglycemic properties, excellently improved liver functions, and efficiently attenuates liver damage via enhancing the hepatic antioxidant capacity, suppressing the hepatic oxidative stress and inflammatory markers. Metformin demonstrated comparatively lower hypoglycemic and hepatic protective efficacy than linagliptin. The combination of these drugs may serve as a novel therapeutic approach for improving liver function in diabetes with fewer adverse effects than monotherapy.
Declarations
Authors’ contributions: Conceptualization: FO, IO; Design and supervision: FO, IO, NO, SO, Performed the experiments with FO’s support. Data Analysis: IO, NO, MO. Writing-original draft: IO, NO, MO. Review: FO, MO, OS. Editing: FO, MO. All authors have read and approved the final manuscript.
- International Diabetes Federation. IDF Diabetes Atlas. 10th ed. Belgium: International Diabetes Federation; 2021. Available from: https://www.diabetesatlas.org
- Banday M, Sameer AS, Nissar S. Pathophysiology of diabetes: An overview. Avicenna J Med. 2020;10(4):174–88. Available from: https://doi.org/10.4103/ajm.ajm_53_20
- Yongxia L, Wang W, Liu J, Xie M, Liu Q, Li S. Vascular Complications of Diabetes: A Narrative Review. Medicine (Baltimore). 2023;102(40):e35285. Available from: https://doi.org/10.1097/md.0000000000035285
- Zhang Q, Lu L, Wang J, Lu M, Liu D, Zhou C, et al. Metabolomic profiling reveals the step-wise alteration of bile acid metabolism in patients with diabetic kidney disease. Nutr Diabetes. 2024;14:85. Available from: https://doi.org/10.1038/s41387-024-00315-0
- Yu MG, Gordin D, Fu J, Park K, Li Q, King GL. Protective Factors and the Pathogenesis of Complications in Diabetes. Endocr Rev. 2024;45:227–52. Available from: https://doi.org/10.1210/endrev/bnad030
- Jiang S, Young JL, Wang K, Qian Y, Cai L. Diabetic induced alterations in hepatic glucose and lipid metabolism: The role of type 1 and type 2 diabetes mellitus (Review). Mol Med Rep. 2020;22(2):603–11. Available from: https://doi.org/10.3892/mmr.2020.11175
- Vakilpour A, Amini-Salehi E, Soltani Moghadam A, Keivanlou MH, Letafatkar N, Habibi A, et al. The effects of gut microbiome manipulation on glycemic indices in patients with non-alcoholic fatty liver disease: a comprehensive umbrella review. Nutr Diabetes. 2024;14(1):25. Available from: https://doi.org/10.1038/s41387-024-00281-7
- Chan WK, Wong VWS, Adams LA, Nguyen MH. MAFLD in adults: non-invasive tests for diagnosis and monitoring of MAFLD. Hepatol Int. 2024;18:909–21. Available from: https://doi.org/10.1007/s12072-024-10661-x
- Amini-Salehi E, Hassanipour S, Joukar F, Daryagasht AA, Khosousi MJ, Aleali MS, et al. Risk factors of non-alcoholic fatty liver disease in the Iranian adult population: a systematic review and meta-analysis. Hepat Mon. 2023;23:e131523. Available from: https://doi.org/10.5812/hepatmon-131523
- Powell EE, Wong VW-S, Rinella M. Non-alcoholic fatty liver disease. Lancet. 2021;397:2212–24. Available from: https://doi.org/10.1016/s0140-6736(20)32511-3
- Naidoo K, Khathi A. Investigating the Effects of Gossypetin on Liver Health in Diet-Induced Pre-Diabetic Male Sprague Dawley Rats. Molecules. 2025;30(8):1834. Available from: https://doi.org/10.3390/molecules30081834
- Lian CY, Zhai ZZ, Li ZF, Wang L. High fat diet-triggered non-alcoholic fatty liver disease: A review of proposed mechanisms. Chem Biol Interact. 2020;330:109199. Available from: https://doi.org/10.1016/j.cbi.2020.109199
- American Diabetes Association. Standards of medical care in diabetes—2020 abridged for primary care providers. Diabetes Care. 2020;43(Suppl 1):S1–212. Available from: https://doi.org/10.2337/cd20-as01
- American Diabetes Association. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes – 2021. Diabetes Care. 2021;44(Suppl 1):S111–24. Available from: https://doi.org/10.2337/dc21-s009
- Ide M, Sonoda N, Inoue T, Kimura S, Minami Y, Makimura H, et al. The dipeptidyl peptidase-4 inhibitor, linagliptin, improves cognitive impairment in streptozotocin-induced diabetic mice by inhibiting oxidative stress and microglial activation. PLoS One. 2020;15:e0228750. Available from: https://doi.org/10.1371/journal.pone.0228750
- Siddiqui N, Ali J, Parvez S, Zameer S, Najmi AK, Akhtar M. Linagliptin, a DPP-4 inhibitor, ameliorates A beta (1-42) peptides induced neurodegeneration and brain insulin resistance (BIR) via insulin receptor substrate-1 (IRS-1) in rat model of Alzheimer’s disease. Neuropharmacology. 2021;195:108662. Available from: https://doi.org/10.1016/j.neuropharm.2021.108662
- Inzucchi S, Bergenstal R, Buse J, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012;55:1577–96. Available from: https://doi.org/10.1007/s00125-012-2534-0
- Arab HH, Eid AH, Mahmoud AM, Senousy MA. Linagliptin mitigates experimental inflammatory bowel disease in rats by targeting inflammatory and redox signaling. Life Sci. 2021;273:119295. Available from: https://doi.org/10.1016/j.lfs.2021.119295
- Tsuprykov O, Ando R, Reichetzeder C, von Websky K, Antonenko V, Sharkovska Y, et al. The dipeptidyl peptidase inhibitor linagliptin and the angiotensin II receptor blocker telmisartan show renal benefit by different pathways in rats with 5/6 nephrectomy. Kidney Int. 2016;89:1049–61. Available from: https://doi.org/10.1016/j.kint.2016.01.016
- Arab HH, Elhemiely AA, El-Sheikh AAK, Khabbaz HJA, Arafa EA, Ashour AM, et al. Repositioning Linagliptin for the Mitigation of Cadmium-Induced Testicular Dysfunction in Rats: Targeting HMGB1/TLR4/NLRP3 Axis and Autophagy. Pharmaceuticals. 2022;15:852. Available from: https://doi.org/10.3390/ph15070852
- Kadowaki T, Wang G, Rosenstock J, Yabe D, Peng Y, Kanasaki K, et al. Effect of linagliptin, a dipeptidyl peptidase-4 inhibitor, compared with the sulfonylurea glimepiride on cardiovascular outcomes in Asians with type 2 diabetes: subgroup analysis of the randomized CAROLINA® trial. Diabetol Int. 2021;12:87–100. Available from: https://doi.org/10.1007/s13340-020-00447-5
- Nirwan N, Vohora D. Linagliptin in Combination With Metformin Ameliorates Diabetic Osteoporosis Through Modulating BMP-2 and Sclerostin in the High-Fat Diet Fed C57BL/6 Mice. Front Endocrinol (Lausanne). 2022;13:944323. Available from: https://doi.org/10.3389/fendo.2022.944323
- Lv Q, Shen J, Miao L, Ye B, Schepers C, Plat A, et al. Early Combination Therapy with Linagliptin and Metformin in People with Type 2 Diabetes Improves Glycemic Control to HbA1c ≤ 6.5% without Increasing Hypoglycemia: Pooled Analysis of Two Randomized Clinical Trials. Diabetes Ther. 2020;11(6):1317–30. Available from: https://doi.org/10.1007/s13300-020-00819-9
- Li Q, Wang X, Guo A, Zheng W, Bi J, He Y, Luo Q. The curative effect of metformin and linagliptin in newly-diagnosed type 2 diabetes patients with non-alcoholic fatty liver disease. Int J Clin Exp Med. 2021;14(1):391–8.
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. Available from: https://doi.org/10.1093/clinchem/18.6.499
- Behzadifar S, Hosseini M, Mohammadnejad J, Asiabanha M. A new colorimetric assay for sensitive detection of glucose-6-phosphate dehydrogenase deficiency based on silver nanoparticles. Nanotechnology. 2021;33(5). Available from: https://doi.org/10.1088/1361-6528/ac2fe5
- Tan X, Testoni G, Sullivan MA, López-Soldado I, Vilaplana F, Gilbert RG, et al. Glycogenin is dispensable for normal liver glycogen metabolism and body glucose homeostasis. Int J Biol Macromol. 2025;291:139084. Available from: https://doi.org/10.1016/j.ijbiomac.2024.139084
- Krishnan B, Ganesan AR, Balasubramani R, Nguyen DD, Chang SW, Wang S, et al. Chrysoeriol ameliorates hyperglycemia by regulating the carbohydrate metabolic enzymes in streptozotocin-induced diabetic rats. Food Sci Hum Wellness. 2020;9(4):346–54. Available from: http://dx.doi.org/10.1016/j.fshw.2020.05.014
- Pouwels S, Sakran N, Graham Y, Leal A, Pintar T, Yang W, et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord. 2022;22(1):63. Available from: https://doi.org/10.1186/s12902-022-00980-1
- Perreault L, Skyler JS, Rosenstock J. Novel therapies with precision mechanisms for type 2 diabetes mellitus. Nat Rev Endocrinol. 2021;17:364–77. Available from: https://doi.org/10.1038/s41574-021-00489-y
- Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, et al. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17):6275. Available from: https://doi.org/10.3390/ijms21176275
- Panelvrushabh S, Kondalkar PP, Polshettiwar GB, Choudhari AT, Vishnu P, Choudhari P. Evaluation of antidiabetic potential, cardio, pancreatic, nephroprotective effect, and herb-herb-drug interaction of Madhukiran formulations in T2 diabetic rats. Phytomed Plus. 2025:100756. Available from: https://doi.org/10.1016/j.phyplu.2025.100756
- Ferdaoussi M. Metabolic and Molecular Amplification of Insulin Secretion. Adv Anat Embryol Cell Biol. 2024;239:117–39. Available from: https://doi.org/10.1007/978-3-031-62232-8_5
- Li J, Yan H, Xiang R, Yang W, Ye J, Yin R, et al. ATP Secretion and Metabolism in Regulating Pancreatic Beta Cell Functions and Hepatic Glycolipid Metabolism. Front Physiol. 2022;13:918042. Available from: https://doi.org/10.3389/fphys.2022.918042
- Casadei G, Filippini M, Brognara L. Glycated Hemoglobin (HbA1c) as a Biomarker for Diabetic Foot Peripheral Neuropathy. Diseases. 2021;9:6. Available from: https://doi.org/10.3390/diseases9010016
- Alatawi KA, Alshubaily FA. Coconut products alleviate hyperglycaemic, hyperlipidimic and nephropathy indices in streptozotocin-induced diabetic wistar rats. Saudi J Biol Sci. 2021;28(8):4224–31. Available from: https://doi.org/10.1016/j.sjbs.2021.06.060
- Faramarzi E, Mehrtabar S, Molani-Gol R, Dastgiri S. The relationship between hepatic enzymes, prediabetes, and diabetes in the Azar cohort population. BMC Endocr Disord. 2025;25:41. Available from: https://doi.org/10.1186/s12902-025-01871-x
- Attia MS, Ayman F, Attia MS, Yahya G, Zahra MH, Khalil MMI, et al. Mitigating diabetes-related complications: Empowering metformin with cholecalciferol and taurine supplementation in type 2 diabetic rats. World J Diabetes. 2024;15(8):1778–92. Available from: https://dx.doi.org/10.4239/wjd.v15.i8.1778
- Bi Y, Yang Y, Yuan X, Wang J, Wang T, Liu Z, et al. Association between liver enzymes and type 2 diabetes: a real world study. Front Endocrinol (Lausanne). 2024;15:1340604. Available from: https://doi.org/10.3389/fendo.2024.1340604
- Tang L, Yang Q, Ma R, Zhou P, Peng C, Xie C, et al. Association between lactate dehydrogenase and the risk of diabetic kidney disease in patients with type 2 diabetes. Front Endocrinol (Lausanne). 2024;15:1369968. Available from: https://doi.org/10.3389/fendo.2024.1369968
- Dore MP, Parodi G, Portoghese M, Pes GM. The Controversial Role of Glucose-6-Phosphate Dehydrogenase Deficiency on Cardiovascular Disease: A Narrative Review. Oxid Med Cell Longev. 2021;2021:5529256. Available from: https://doi.org/10.1155/2021/5529256
- Çelik R, Mert H, Comba B, Mert N. Effects of cinnamaldehyde on glucose-6-phosphate dehydrogenase activity, some biochemical and hematological parameters in diabetic rats. Biomarkers. 2022;27(3):270–7. Available from: https://doi.org/10.1080/1354750X.2022.2032351
- Fuentes-Lemus E, Usgame K, Fierro A, López-Alarcón C. Enzymes of glycolysis and the pentose phosphate pathway as targets of oxidants: Role of redox reactions on the carbohydrate catabolism. Redox Biochem Chem. 2025;11:100049. Available from: https://doi.org/10.1016/j.rbc.2025.100049
- Mustafa I, Anwar H, Irfan S, Muzaffar H, Ijaz MU. Attenuation of carbohydrate metabolism and lipid profile by methanolic extract of Euphorbia helioscopia and improvement of beta cell function in a type 2 diabetic rat model. BMC Complement Med Ther. 2022;22:23. Available from: https://doi.org/10.1186/s12906-022-03507-2
- Katz A. The role of glycogen phosphorylase in glycogen biogenesis in skeletal muscle after exercise. Sports Med Health Sci. 2023;5(1):29–33. Available from: https://doi.org/10.1016/j.smhs.2022.11.001
- Kelley DE, Mandarino LJ. Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus. J Clin Invest. 1990;86:1999–2007. Available from: https://doi.org/10.1172/jci114935
- Govindarajan S, Babu SN, Vijayalakshmi MA, Manohar P, Noor A. Aloe vera carbohydrates regulate glucose metabolism through improved glycogen synthesis and down-regulation of hepatic gluconeogenesis in diabetic rats. J Ethnopharmacol. 2021;281:114556. Available from: https://doi.org/10.1016/j.jep.2021.114556
- Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122:4–12. Available from: https://doi.org/10.1172/jci60016
- Kalra S, Raizada N. Dyslipidemia in diabetes. Indian Heart J. 2024;76(1):S80–S82. Available from: https://doi.org/10.1016/j.ihj.2023.11.002
- Hoekstra M, Van Eck M. High-density lipoproteins and non-alcoholic fatty liver disease. Atheroscler Plus. 2023;53:33–41. Available from: https://doi.org/10.1016/j.athplu.2023.08.001
- Komorizono Y, Hosoyamada K, Imamura N, Kajiya S, Hashiguchi Y, Ueyama N, et al. Metformin dose increase versus added linagliptin in non-alcoholic fatty liver disease and type 2 diabetes: an analysis of the JLINK study. Diabetes Obes Metab. 2021;23(3):832–7. Available from: https://doi.org/10.1111/dom.14263
- Lin YY, Weng SF, Hsu CH, Huang CL, Lin YP, Yeh MC, et al. Effect of metformin monotherapy and dual or triple concomitant therapy with metformin on glycemic control and lipid profile management of patients with type 2 diabetes mellitus. Front Med (Lausanne). 2020;9:995944. Available from: https://doi.org/10.3389/fmed.2022.995944
- Obafemi TO, Jaiyesimi KF, Olomola AA, Olasehinde OR, Olaoye OA, Adewumi FD, et al. Combined effect of metformin and gallic acid on inflammation, antioxidant status, endoplasmic reticulum (ER) stress and glucose metabolism in fructose-fed streptozotocin-induced diabetic rats. Toxicol Rep. 2021;8:1419–27. Available from: https://doi.org/10.1016/j.toxrep.2021.07.011
- Palsamy P, Sivakumar S, Subramanian S. Resveratrol attenuates hyperglycemia-mediated oxidative stress, proinflammatory cytokines and protects hepatocytes ultrastructure in streptozotocin-nicotinamide-induced experimental diabetic rats. Chem Biol Interact. 2010;186(2):200–10. Available from: https://doi.org/10.1016/j.cbi.2010.03.028
- Zeng F, Luo J, Han H, Xie W, Wang L, Han R, et al. Allopurinol ameliorates liver injury in type 1 diabetic rats through activating Nrf2. Int J Immunopathol Pharmacol. 2021;35:1–13. Available from: https://doi.org/10.1177/20587384211031417
- Xi J, Wang S, Chen J, Law JC, Fan Z, Lv G. The role of C-reactive protein to lymphocyte ratio in NAFLD and mortality among NAFLD patients. BMC Gastroenterol. 2025;25:327. Available from: https://doi.org/10.1186/s12876-025-03924-w
- Lodhi S, Vadnere GP, Patil KD, Patil TP. Protective effects of luteolin on injury induced inflammation through reduction of tissue uric acid and proinflammatory cytokines in rats. J Tradit Complement Med. 2020;10(1):60–9. Available from: https://doi.org/10.1016/j.jtcme.2019.02.004