More Information
Submitted: March 27, 2025 | Approved: April 11, 2025 | Published: April 12, 2025
How to cite this article: Amor IB, Frikha I, Medhaffer M, Elloumi M. Gilbert’s Syndrome Revealed by Hepatotoxicity of Imatinib. Ann Clin Gastroenterol Hepatol. 2025; 9(1): 001-003. Available from:
https://dx.doi.org/10.29328/journal.acgh.1001049.
DOI: 10.29328/journal.acgh.1001049
Copyright License: © 2025 Amor IB, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Gilbert’s syndrome; Imatinib; Hepatotoxicity; UGT1A1 polymorphism; Chronic myeloid leukemia
Gilbert’s Syndrome Revealed by Hepatotoxicity of Imatinib
Imen Ben Amor1,2*, Imen Frikha1,2, Moez Medhaffer1,2 and Moez Elloumi1,2
1Department of Hematology, Hedi Chaker University Teaching Hospital, Sfax, Tunisia
2Faculty of Medicine, University of Sfax, Sfax, Tunisia
*Address for Correspondence: Imen Ben Amor, Department of Hematology, Faculty of Medicine, University of Sfax, Sfax, Tunisia, Email: [email protected]
Gilbert’s Syndrome (GS) is a hereditary disease that can cause hyperbilirubinemia due to a mutation in the promoter of the UGT1A1 gene, which causes a decrease in uridine diphosphate glucuronyltransferase enzyme activity. Polymorphisms in the UGT1A1 gene are associated with induced hyperbilirubinemia by Tyrosine Kinase Inhibitors (TKI) in Chronic Myeloid Leukemia (CML).
We report a case of patient who developed hepatotoxicity when treated on Imatinib and subsequently diagnosed with Gilbert’s syndrome. Eight months after initiating Imatinib, the patient developed conjunctival jaundice and signs of hepatotoxicity with increase in liver enzymes and hyperbilirubinemia with elevated level of unconjugated bilirubin. Gilbert’s syndrome was suspected in the presence of predominantly unconjugated hyperbilirubinemia and a prior history of transient episodes of jaundice. Genetic testing revealed homozygosity for the UGT1A1 TA7 (*28) polymorphism. Imatinib was stopped due to continuous increase of aminotransferases and hyperbilirubinemia and restarted after improvement of Liver Function Tests (LFTs) with a reduced dose of 200 mg/day but LFTs worsted again, and the patient was switched to Dasatinib 100 mg/day, without hepatic cytolysis and a mild persistent hyperbilirubinemia after a follow up of 20 months.
Patients with an unexplained rise in serum bilirubin levels on Imatinib therapy should be screened for the genetic UGT1A1 polymorphisms.
Gilbert Syndrome (GS) is a common genetic disorder affecting bilirubin metabolism and causing unconjugated hyperbilirubinemia with otherwise normal transaminases and liver function tests, due to a mutation in the promoter of the UGT1A1 gene, coding the enzyme bilirubin uridine diphosphate glucuronosyltransferase (UGT-1A), which plays a key role in the bilirubin metabolism [1].
Gilbert’s syndrome is mainly associated to UGT1A1*28 variant, which is characterized by the insertion of Thymine-Adenine (TA) in the UGT promoter region, forming the 7/7 polymorphism [2]. Additionally, polymorphisms in the UGT1A1 gene are associated with induced hyperbilirubinemia by Tyrosine Kinase Inhibitors (TKI) in Chronic Myeloid Leukemia (CML) patients, and Imatinib, the TKI commonly used in frontline therapy in CML, has been shown to have inhibitory activity against UGT1A1 [3].
We report a case of Gilbert syndrome diagnosed in a patient who developed hepatotoxicity when treated with Imatinib.
An 18-year-old male was followed for CML in chronic phase. The patient started treatment with Imatinib (400 mg/day) with baseline normal Liver Function Tests (LFTs) before initiation. Liver Function Tests (LFTs) were monitored monthly. Eight months after starting treatment, the laboratory tests show mild elevation in liver enzymes: aspartate aminotransferase (AST48 U/L, (normal up to 40) and alanine aminotransferase (ALT: 63 U/l (normal up to 45). Fifteen days after the patient developed conjunctival jaundice and LFTs continued to increase with AST: 87U/L and ALT: 172U/L, hyperbilirubinemia 25 mg/l (normal up to 14 mg/l) with elevated level of unconjugated bilirubin 23 mg/l (normal up to 11 mg/l). Notably, alkaline phosphatase and gamma-glutamyl transferase levels remained within normal limits.
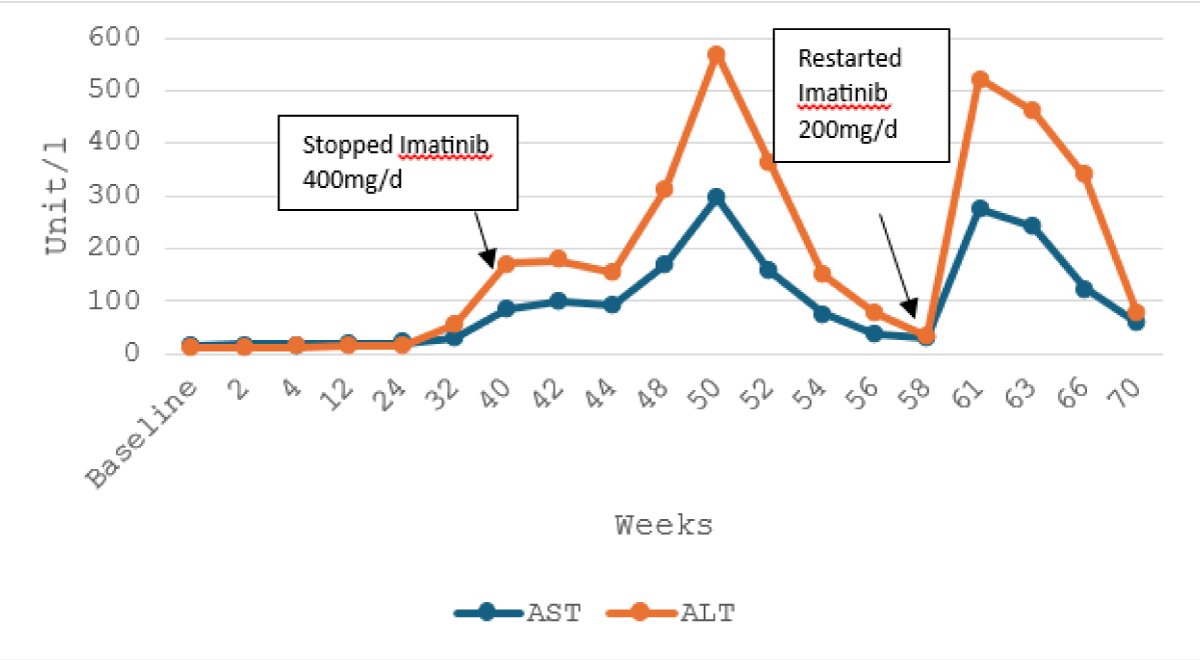
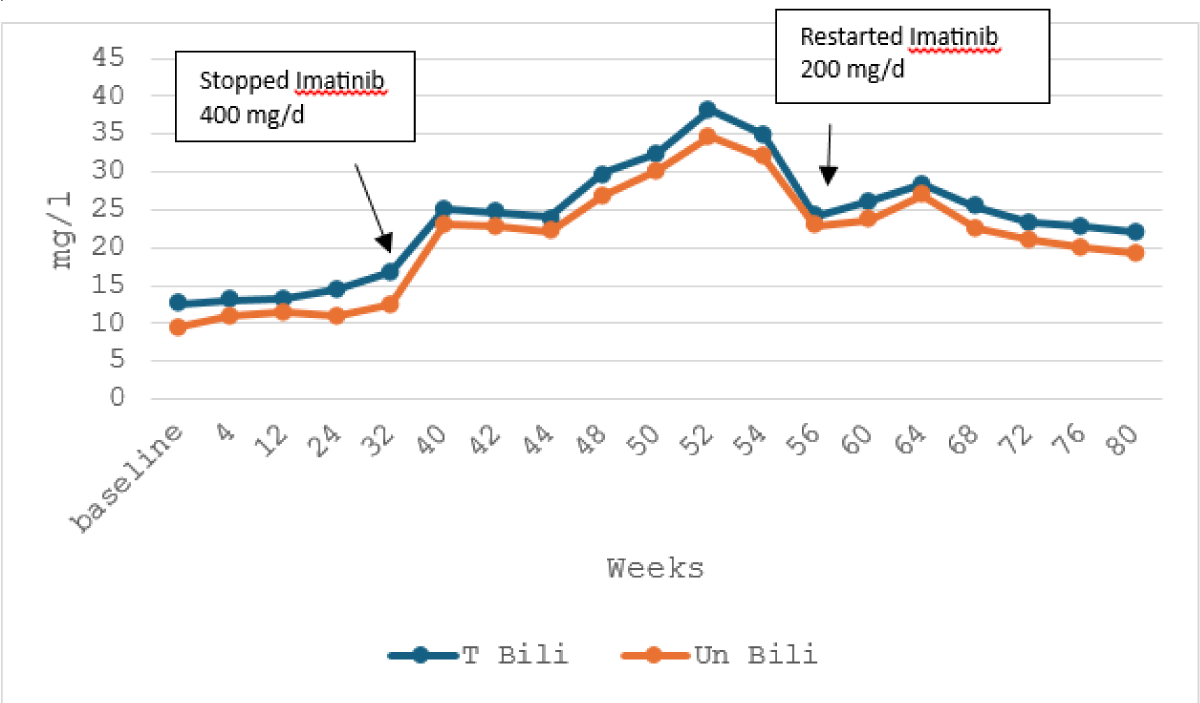
An abdominal ultrasound revealed no abnormalities, and viral hepatitis screening for hepatitis A, B, and C was negative. Imatinib was discontinued, but blood tests revealed further deterioration in LFTs with a peak after 10 weeks, AST 296 U/L, ALT: 570 U/l, and an increase of hyperbilirubinemia: 38,8 mg/l and unconjugated bilirubin: 34,6 mg/l.
In the presence of predominantly unconjugated hyperbilirubinemia, and a prior history of transient episodes of jaundice reported by the patient’s parents, Gilbert’s syndrome was suspected.
Genetic analysis of the UGT1A1 gene was performed by PCR amplification and Sanger sequencing, and the patient was tested positive for the UGT1A1 TA7 (*28) genotype as homozygous for the TA7 polymorphism.
Four months after imatinib discontinuation, aminotransferases returned to normal levels with mild increase of bilirubin. Then, we restart Imatinib with a reduced dose of 200 mg/day but LFTs worsened again (Figures 1,2).
Figure 1: Trend of alanine and aspartate aminotransferase with the course of imatinib therapy.
Figure 2: Trend of total and unconjugated bilirubin with the course of imatinib therapy.
Owing to recurrent severe hepatotoxicity during Imatinib therapy, treatment was transitioned to Dasatinib 100 mg/day, without hepatic cytolysis and a mild persistent hyperbilirubinemia after a follow-up of 20 months.
Imatinib is the first Tyrosine Kinase Inhibitor (TKI) approved for the treatment of chronic myeloid leukemia (CML) [4].
Hepatotoxicity is a serious adverse event associated with Imatinib, seen in 5% in randomized phase III trials of patients with CML treated with the drug [5].
In a recent meta-analysis, hepatotoxicity adverse events were reported in 12,6% of patients (n = 1268/10046), with 64.7% grade I and II, and 28.6% grade III and IV adverse events [6].
Gilbert’s syndrome is the most common hereditary cause of mild unconjugated hyperbilirubinemia, and approximately 3% - 10% of the population is estimated to have Gilbert’s syndrome based on serum bilirubin levels [7].
The variant UGT1A1 TA7 (*28), the case of our patient, is detected in the majority of patients [2]. In a Turkish study, it was identified in 75.5% of cases [8].
Drug-induced hyperbilirubinemia has been reported in patients with the A(TA)7TAA polymorphism treated with TKIs, particularly Nilotinib which is a potent non-competitive inhibitor of the enzyme UGT1A1 inducing hyperbilirubinemia in patients with CML [9,10].
A study investigating the inhibitory effects of TKIs on UDP-Glucuronosyltransferase (UGT) activities showed that Imatinib was an intermediate inhibitor of UGT1A1 in vitro [3].
Another study including patients having genetic UGT1A1 polymorphisms and treated with 3 different TKIs, showed hyperbilirubinemia with the 3 TKIs, but it was more frequent in patients on nilotinib (44%), compared to imatinib (14%) and dasatinib (8%), especially with the 7/7 genotype [11].
A case series reported 2 patients treated on Imatinib for GIST, who were diagnosed with UGT1A1 (*28) polymorphism after Imatinib induced hyperbilirubinemia. Treatment was discontinued in one patient due to severe and persistent hyperbilirubinemia, despite a reduced dose of the Imatinib to 100 mg every other day, while the other patient improved at this dose [12].
In our case, Imatinib was restarted with a reduced dose of 200mg/day, but LTFs worsened again with the increase in bilirubinemia. Switched treatment on Dasatinib did not cause hepatic cytolysis and did not worsen hyperbilirubinemia.
We report a case of a CML patient treated with Imatinib who was subsequently diagnosed with Gilbert’s syndrome.
Usually, individuals with Gilbert’s Syndrome are asymptomatic and do not require specific treatment due to the benign nature of the condition.
However, the use of some TKIs in patients with UGT1A1 polymorphism may lead to the development of hyperbilirubinemia, which can lead to the worsening of Gilbert’s Syndrome.
Patients with an unexplained rise in serum bilirubin levels on Imatinib therapy should be screened for the genetic UGT1A1 polymorphisms.
Our case illustrates the coexistence of two rare conditions that could cause hyperbilirubinemia, in the absence of bilirubin alteration, however, we cannot draw conclusions from a single case.
Reporting similar cases will help raise clinical awareness and identify secondary conditions that could worsen the course of treatment.
These data on imatinib-induced Gilbert’s syndrome may prompt a similar FDA requirement, for the testing of the UGT1A1 polymorphism, as is already done for nilotinib
Authors’ contributions: All authors contributed to the manuscript.
- Fretzayas A, Moustaki M, Liapi O, Karpathios T. Gilbert syndrome. Eur J Pediatr. 2012;171(1):11-15. Available from: https://doi.org/10.1007/s00431-011-1641-0
- Mi X, Yan J, Ma X, Zhu G, Gao Y, Yang W, et al. Analysis of the UGT1A1 genotype in hyperbilirubinemia patients: differences in allele frequency and distribution. Biomed Res Int. 2019;2019:6272174. Available from: https://doi.org/10.1155/2019/6272174
- Zhang N, Liu Y, Jeong H. Drug-drug interaction potentials of tyrosine kinase inhibitors via inhibition of UDP-glucuronosyltransferases. Sci Rep. 2015;5:17778. Available from: https://doi.org/10.1038/srep17778
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031-1037. Available from: https://doi.org/10.1056/nejm200104053441401
- Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408-2417. Available from: https://doi.org/10.1056/nejmoa062867
- Tobaiqy M, Helmi N, MacLure K, Saade S. The prevalence of hepatic and thyroid toxicity associated with imatinib treatment of chronic myeloid leukaemia: a systematic review. Int J Clin Pharm. 2024;46(2):368-381. Available from: https://doi.org/10.1007/s11096-023-01671-0
- Rasool A, Sabir S, Ashlaq M, Farooq U, Khan MZ, Khan FY. Gilbert’s syndrome - a concealed adversity for physicians and surgeons. J Ayub Med Coll Abbottabad. 2015;27(3):707-710. Available from: https://pubmed.ncbi.nlm.nih.gov/26721045/
- Appak YÇ, Aksoy B, Özyılmaz B, Özdemir TR, Baran M. Gilbert syndrome and genetic findings in children: a tertiary-center experience from Turkey. Turk Arch Pediatr. 2022;57(3):295-299. Available from: https://doi.org/10.5152/TurkArchPediatr.2022.21291
- Fujita K, Sugiyama M, Akiyama Y, Ando Y, Sasaki Y. The small-molecule tyrosine kinase inhibitor nilotinib is a potent noncompetitive inhibitor of the SN-38 glucuronidation by human UGT1A1. Cancer Chemother Pharmacol. 2011;67(1):237-241. Available from: https://doi.org/10.1007/s00280-010-1445-3
- Singer JB, Shou Y, Giles F, Kantarjian HM, Hsu Y, Robeva AS, et al. UGT1A1 promoter polymorphism increases risk of nilotinib-induced hyperbilirubinemia. Leukemia. 2007;21(11):2311-2315. Available from: https://doi.org/10.1038/sj.leu.2404827
- Bahk J, Claudiani S, Szydlo RM, Toma S, Abdillah F, Hing S, et al. The association of Gilbert’s syndrome with hyperbilirubinaemia occurring on any of imatinib, dasatinib and nilotinib in patients with chronic myeloid leukaemia (CML). Blood. 2015;126(23):2795. Available from: https://doi.org/10.1182/blood.V126.23.2795.2795
- Saif MW, Smith MH, Maloney A, Diasio RB. Imatinib-induced hyperbilirubinemia with UGT1A1 (*28) promoter polymorphism: first case series in patients with gastrointestinal stromal tumor. Ann Gastroenterol. 2016;29(4):551-556. Available from: https://doi.org/10.20524/aog.2016.0053