More Information
Submitted: January 03, 2024 | Approved: January 17, 2024 | Published: January 18, 2024
How to cite this article: Kadi N, Chowdhury A, Hanks M, Zaitoun AM. Case Series of Metastatic Cutaneous Malignant Melanoma to the Gallbladder and the First Reported Case of Metachronous Adenocarcinoma of the Colon. Ann Clin Gastroenterol Hepatol. 2024; 8: 001-005.
DOI: 10.29328/journal.acgh.1001044
Copyright License: © 2024 Kadi N, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Melanoma; Metastasis; Gallbladder; BRAF; Metachronous adenocarcinoma
Case Series of Metastatic Cutaneous Malignant Melanoma to the Gallbladder and the First Reported Case of Metachronous Adenocarcinoma of the Colon
Nourdin Kadi1, Abid Chowdhury2, Matthew Hanks1 and Abed M Zaitoun1*
1Department of histopathology, Nottingham University Hospital NHS Trust, Nottingham, UK
2Department of Surgery, Nottingham University Hospital NHS Trust, Nottingham, UK
*Address for Correspondence: Lucio Abed M Zaitoun, Department of histopathology, Nottingham University Hospital NHS Trust, Nottingham, UK, Email: [email protected]
Two female patients in their fifties with a previous history of cutaneous malignant melanoma were found during follow-up to have a 'hot' lesion in the gallbladder on a Positron Emission Tomography scan. Imaging showed a gallbladder polyp. Histology revealed infiltration of the polyp mucosa by metastatic malignant melanoma. One case had a BRAF mutation.
A male in his 70s was found on a staging computed tomography scan to have a suspicious intraluminal lesion in the gallbladder and thickening of the sigmoid colon. Subsequent histology confirmed metastatic malignant melanoma in the chest wall and to the gallbladder and adenocarcinoma in the colon. Molecular testing showed BRAF mutation. The metachronous adenocarcinoma in the colon was mismatch repair protein proficient and had no KRAS mutation. Histology from all cases showed that metastatic malignant melanoma to the gallbladder is superficial.
Discussion: Reports from autopsy examinations revealed that metastasis from malignant melanoma to the gallbladder can be up to 15% - 20%. Most patients have mild symptoms or are asymptomatic which explains the paucity of cases reported in living patients within the published literature. Most of the previous reports showed the metastatic malignant melanoma to the gallbladder presented macroscopically as a polyploidal lesion.
Conclusion: Our histological observation and previous reports showed that metastatic malignant melanoma in the gallbladder tends to be superficial. All our cases show no lymphatic or vascular invasion in the histological examination as previously published reports, however, the hematological spread is the most commonly suggested mechanism of spread.
Melanoma is the fifth most common cancer in the United Kingdom with 16744 new cases of melanoma skin cancer diagnosed in the UK during 2018. It is well known that metastasis from cutaneous Malignant Melanoma (MM) can affect any organ of the body. 2% - 4% of patients affected by cutaneous MM are diagnosed with gastrointestinal metastases [1-3]. The gall bladder is one of the most common sites for metastases for cutaneous MM [4,5].
We report a cases series of metastatic cutaneous MM in the gallbladder including a case of metachronous metastatic cutaneous MM of the gallbladder and adenocarcinoma of the colon.
Case 1
A 53-year-old female, with a previous history of cutaneous MM Clark level IV in 2014, was found to have a new ‘hot’ lesion in the gallbladder bed on a follow-up Positron Emission Tomography (PET) scan 2 years later. Computed Tomography (CT) scan and Magnetic Resonance Imaging (MRI) of the liver confirmed the presence of a gallbladder polyp. Serum liver function tests and urea and electrolytes were within normal ranges. The case was discussed at a Multi-Disciplinary Team (MDT) meeting and she underwent a cholecystectomy with wedge resection of the liver and regional lymph node dissection. Macroscopic examination of the gallbladder confirmed a gallbladder polyp. However, histological examination showed an ulceration polyp and the mucosa infiltrated by tumor cells with prominent nucleoli and atypical mitosis. Immunohistochemistry using S100, Melan-A, and HMB45 confirmed a melanocytic origin. The histology was interpreted as metastatic malignant melanoma to the gallbladder. Subsequent molecular studies revealed a BRAF V600 mutation on Exon 15.
Case 2
A 58-year-old female with a previous history of cutaneous malignant melanoma in 2016 with a Clark level IV was also found to have a lesion in the gallbladder on a PET scan during follow-up 3 years later. An enhanced CT scan and liver MRI confirmed a gallbladder polyp. Similarly, the serum liver function tests, urea and were within normal ranges. Following a discussion at an MDT meeting, she underwent a cholecystectomy with wedge resection of the liver and regional lymphadenectomy. A macroscopic examination of the gallbladder confirmed a gallbladder polyp. Histological examination showed a polyp with the mucosa infiltrated by tumor cells containing prominent nucleoli. Immunohistochemistry for S100, Melan A, and HMB45 was performed which confirmed the melanocytic origin of the tumor cells. The case was reported as metastatic malignant melanoma to the gallbladder; molecular testing was not available at the time of the diagnosis.
Case 3
A 73-year-old male was diagnosed with recurrent cutaneous malignant melanoma of the left upper abdominal skin in late 2019. The Breslow thickness was 9.1mm and Clark level IV. Histology noted that tumour cells were close to the excision margin prompting discussion at an MDT meeting; a wide local excision and close follow-up were recommended. Subsequent histological examination confirmed no residual malignancy.
The patient had a CT scan at 6 months following the wide local excision which showed a suspicious left axillary lymph node. Histology confirmed metastatic cutaneous malignant melanoma. The case was re-discussed at MDT and the patient was commenced on PD-1 immunotherapy (pembrolizumab).
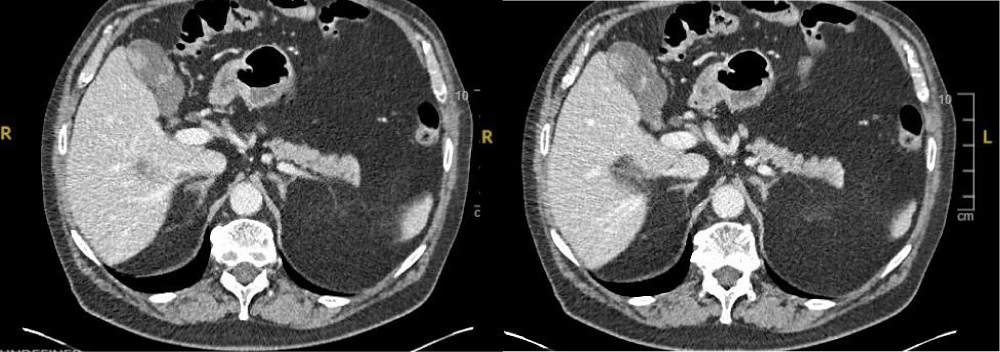
At 23 months follow up he reported a right upper quadrant discomfort. Clinical examination revealed a suspicious skin lesion on the chest wall. Serum liver function tests and urea and electrolytes showed no significant abnormalities. However, a CT scan with contrast (Figure 1) revealed a suspicious lesion in the gallbladder and incidental thickening of the sigmoid colon which was later diagnosed as moderately differentiated adenocarcinoma of the sigmoid colon.
Figure 1: CT scan showed a suspicious intra-luminal lesion in the gallbladder.
Frozen section
During surgery for the sigmoid adenocarcinoma and gallbladder, a nodule in the peritoneum and liver nodule was identified. Both were sent for a frozen section before proceeding with the surgery.
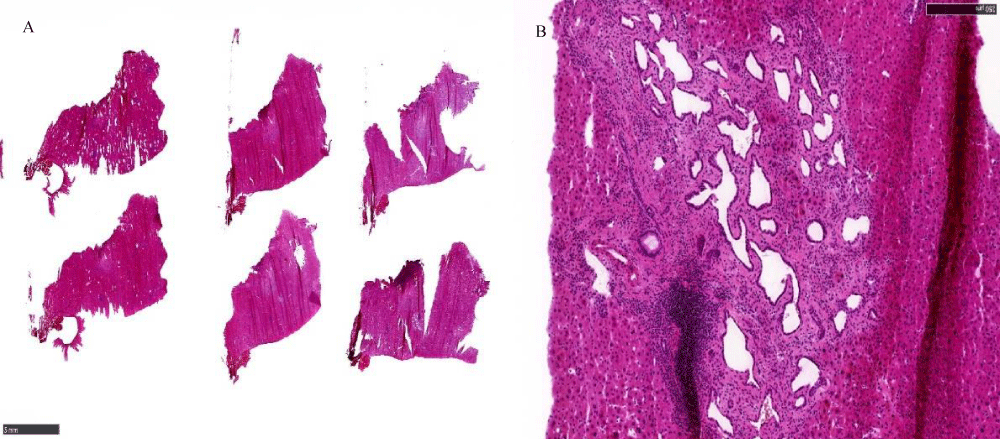
The frozen section was reported as fibrous tissue and no metastatic disease for the peritoneum nodule. (Figure 2A). The liver nodule was reported as biliary hamartoma (Figure 2B).
Figure 2: A: Fibrous tissue on frozen section. B: Biliary Hamartoma on frozen section.
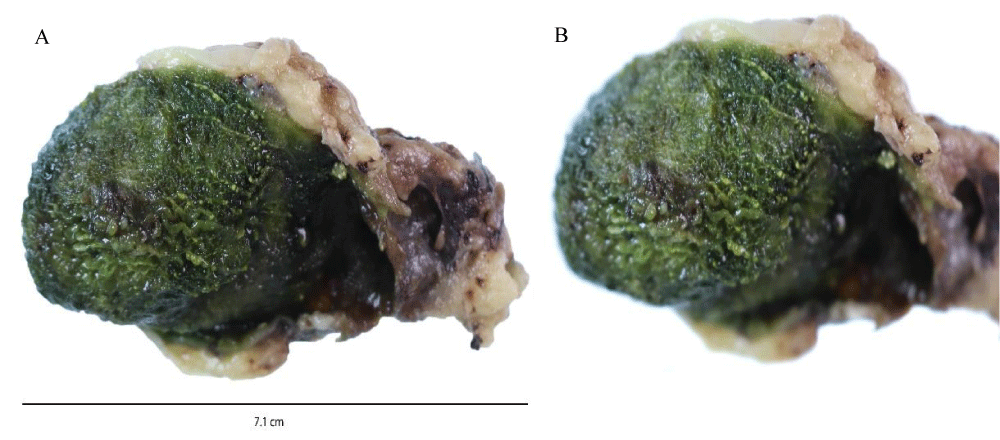
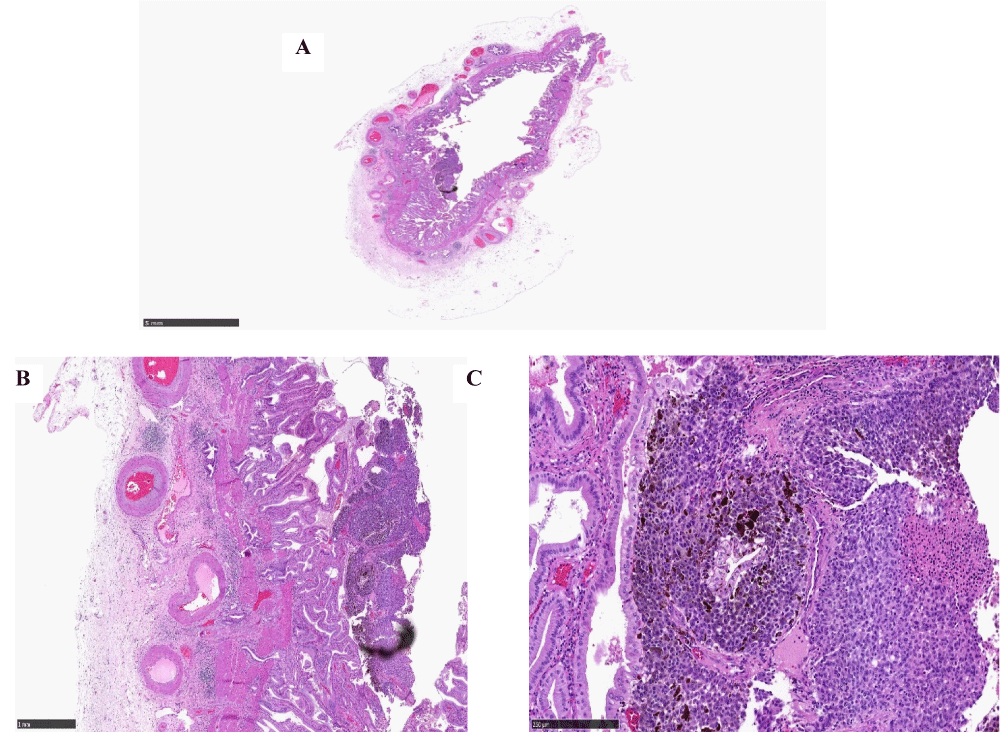
Macroscopic examination for case 3: Macroscopic examination of the gallbladder revealed a mild thickening of the mucosa as shown in Figures 3A, 3B.
Figure 3: A: Macroscopic Appearance of gallbladder including mucosal thickening. B: Magnified area of mucosal thickening.
Final histology for case 3: Histological examination confirmed the frozen section diagnosis of fibrous tissue for the peritoneum nodule and biliary hamartoma for the liver nodule.
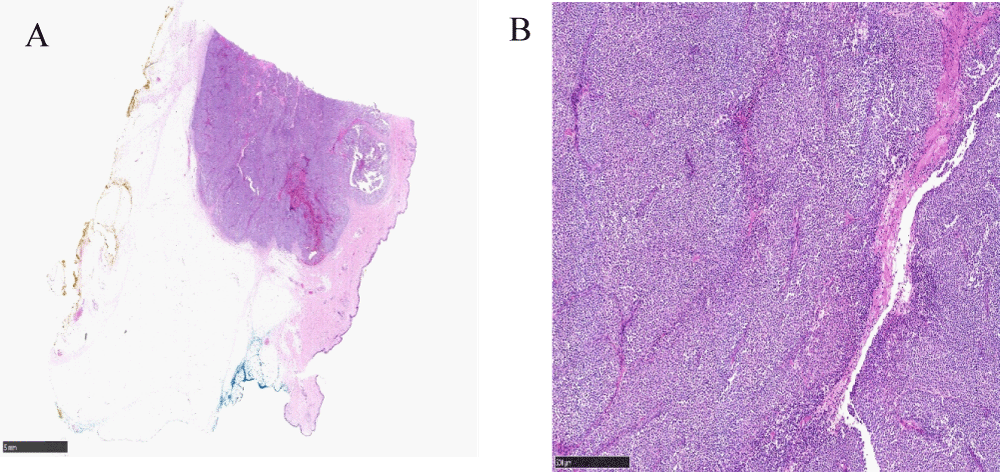
Histological examination of the chest lesion confirmed metastatic cutaneous malignant melanoma in the chest wall as shown in Figures 4A, 4B.
Figure 4: A: Low-power view of cutaneous malignant melanoma in the chest wall (x2 magnification). B: High power view of cutaneous malignant melanoma in the chest wall (x20 magnification).
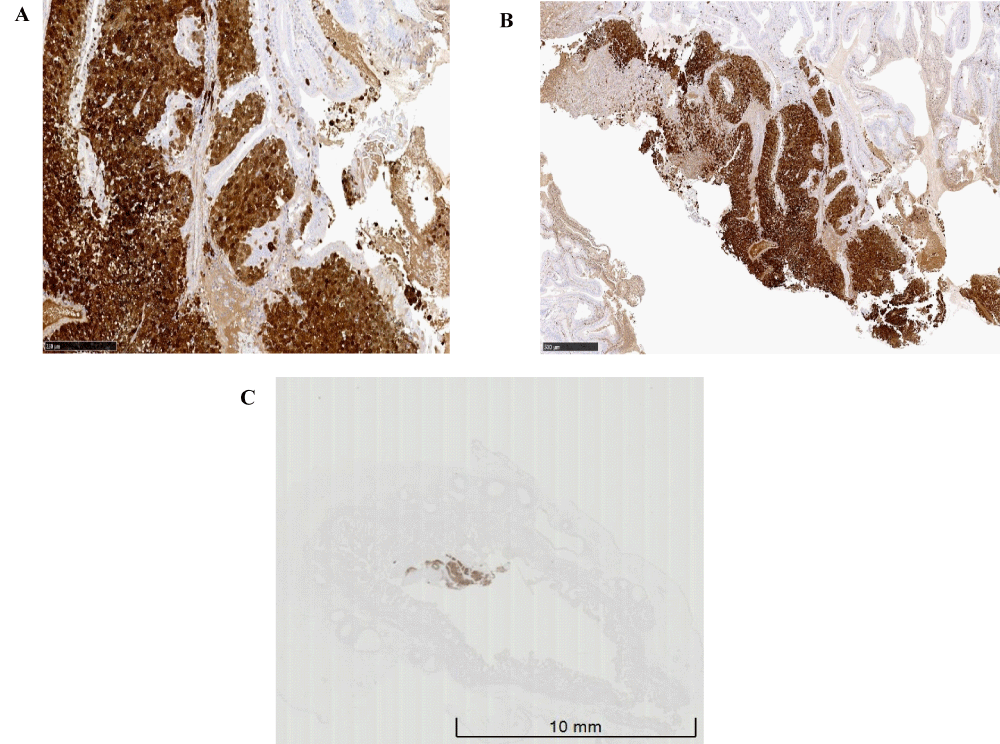
Histology for the thickened area in the gallbladder showed mucosa infiltrated by tumor cells and melanin pigment (Figure 5A-5C). No invasion beyond gallbladder mucosa was identified. There was no lymphatic or vascular invasion. Immunohistochemistry was performed including S100, HMB45, and Melan A as shown in Figure 6 which confirmed the diagnosis of metastatic cutaneous MM to the gallbladder. Molecular testing for BRAF mutation showed both metastatic cutaneous malignant melanoma lesions have BRAF V600 mutation in Exon 15.
Figure 5: A: Low power view of cutaneous malignant melanoma in the chest wall (x2 magnification), B: Medium power view of cutaneous malignant melanoma in the chest wall (x20 magnification), C: High power view of cutaneous malignant melanoma in the chest wall (x20 magnification).
Figure 6: A: Positive S100 Immunohistochemistry, B: Positive Melan-A Immuno-histochemistry, C: Positive HMB-45 Immunohistochemistry.
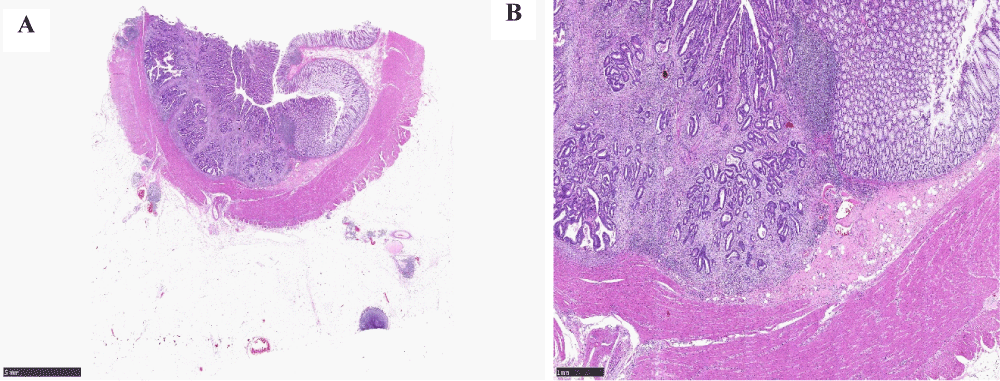
The histological examination of the colonic lesion showed a moderately differentiated adenocarcinoma (Figures 7A,7B). The adenocarcinoma was Mismatch Repair (MMR) proficient and there was no KRAS mutation.
Figure 7: A. Low power view of the colonic adenocarcinoma (x2 magnification), B: High power view of the colonic adenocarcinoma (x20 magnification).
Malignant Melanoma is the 5th most common cancer in the UK, accounting for 4% of all new cancer cases1. Metastasis malignancy to the gallbladder is rare. Das Gupta and Brasfield in 1964 reported 125 autopsied cases of metastatic malignant melanoma and found a 15% incidence of gallbladder metastasis [4]. Most patients have mild symptoms or are asymptomatic which explains the paucity of cases reported in living patients in the published literature [4].
Despite the belief by most investigators that malignant melanoma of the gallbladder is secondary to cutaneous, retinal, or meningeal melanoma [5-7] some investigators have proposed that primary malignant melanoma can and does arise in the gallbladder [8-11]. Whether or not primary malignant melanoma of the gallbladder can occur is still in serious doubt [12]. In our series, the malignant melanoma in the gallbladder is metastatic from the cutaneous primary. The mean age of diagnosis in the published literature was 53.4 (range 35–77). In our series, the mean age is 62 years which is slightly higher than the average reported age. Fewer than half of the documented cases in published literature included detailed histological records of the primary lesions [14]. Of those that did Clark level is IV most common [13]. In our series primary cutaneous malignant melanoma Clark level was IV.
The majority of previous case reports show that metastatic malignant melanoma to the gallbladder presented macroscopically as a polyploid lesion and infrequently as a flat lesion [13-16]. In our series, two cases presented as a polypoid lesion and there was a gallbladder mucosa thickening in the third case. Some authors argue that the use of ultrasound examination may aid in the detection of gallbladder metastasis [13]. Ultrasound imaging is operator dependant and flat lesion identification may be very difficult compared to other imaging modalities such as PET or CT. Follow-up imaging is highly recommended when patients have poor histological prognostic parameters.
All our cases had cholecystectomy with wedge resection of the liver. This methodology provides a specimen for histopathological assessment and molecular studies. Knowing the mutation status will guide future treatment options. All the cases were operable with imaging reported as highly suspicious for metastatic disease however in inoperable cases it may be beneficial after a multi-disciplinary team meeting to consider Conclusion, and analyzing the molecular status of the lesion. We agree with Al-Janabi and colleagues that despite the lack of standard guidelines for the treatment of metastatic melanoma, surgical resection remains the procedure of choice in case of resectable lesions [17-19].
Prognostic factors for primary cutaneous melanomas are still debatable [14], however, most authors use the following parameters: Breslow thickness, ulceration Clark level, lymphovascular invasion, lymph node status, mitotic index growth face, perineural invasion, tumor regression, mitotic index, tumor-infiltrating lymphocytes activity, Satellite/microsatellite/in-transit metastases, location of the primary tumor and TNM stage [14].
Our histological observation revealed that metastatic cutaneous malignant melanoma in the gallbladder is limited to the mucosa. Doole and Higgins also noticed that metastatic melanoma was also confined to the mucosa [15,16]. Most case reports did not show lymphatic or vascular invasion in histological examination [15,16,20,21], however, the hematological spread of malignant melanoma to the gallbladder is the most commonly suggested mechanism of spread continues to be postulated as the likely mechanism of spread [20,21]. Further work is likely to be needed to understand the mechanism of metastasis which might help in developing new treatments and preventing metastases of malignant melanoma.
We searched the MEDLINE database using a combination of “metastatic malignant melanoma” “gall bladder” AND “adenocarcinoma of the colon”. No previous report of metachronous metastatic malignant melanoma to the gallbladder and adenocarcinoma of the colon has been found in published literature and we are reporting the first case.
Molecular studies play an increasing role in the treatment and prognosis of MM. Most previous reports showed that metastatic malignant melanoma to the gallbladder has a BRAF mutation when it was checked [22,23]. This was also the case in our series, two of our cases had BRF mutation checked and there was BRAF V600 mutation. Furthermore, MM with BRAF mutation is suitable for targeted therapy with BRAF inhibitor drugs. That might also be of prognostic value.
Our patient with a metachronous adenocarcinoma in the colon showed MMR proficiency and no KRAS mutation. Further studies are likely to be needed to help characterize the observed phenomenon.
Metastasis from malignant melanoma can affect any organ of the body. Patients with a history of malignant melanoma with histological features suggestive of aggressive behavior should have routine follow-up imaging for the gallbladder in order to detect metastasis. Previous suggestions of US scan imaging might miss flat lesions therefore PET scan, enhanced CT scan or MRI liver are better modalities.
Cholecystectomy with wedge resection of the liver remains the initial treatment for resectable lesions. This approach enables histological and molecular assessment.
We found from our series and previous reports that metastatic malignant melanoma is confined to the mucosa. Further work is needed to understand why metastatic malignant melanoma to the gallbladder is confined to the mucosa which might help in developing new treatment and preventing metastases.
From our cases and previous reports, we found that the majority of metastatic malignant melanoma to gallbladder has BRAF mutation which is important to know eligibility to BRAF target therapy and could predict prognosis. The patients in our case series had previous metastatic disease so were under routine monitoring. We believe that ultrasound scanning is operator-dependent and flat lesions in the gall bladder can easily be overlooked; given this experience, our Trust opts to follow up with patients using a CT or PET methodology.
Metastatic malignant melanoma to the gallbladder with metachronous carcinoma is extremely rare and to the best of our knowledge, we reported the first case of metachronous metastatic cutaneous malignant melanoma of the gallbladder and adenocarcinoma of the colon. This adenocarcinoma in the colon showed MMR proficiency and no KRAS mutation
Ethical considerations
To preserve patient confidentiality, we have omitted any data that may be identifiable to individual patients. No ethical approval was required for the publication.
- CRUK. Melanoma incidence statistics. 2018. http://www.cancerresearchuk.org/cancer-info/cancerstats/types/skin/incidence/
- Giannini I, Cutrignelli DA, Resta L, Gentile A, Vincenti L. Metastatic melanoma of the gallbladder: report of two cases and a review of the literature. Clin Exp Med. 2016 Aug;16(3):295-300. doi: 10.1007/s10238-015-0353-6. Epub 2015 May 1. PMID: 25929736.
- Ettahri H, Elomrani F, Elkabous M, Rimani M, Boutayeb S, Mrabti H, Errihani H. Duodenal and gallbladder metastasis of regressive melanoma: a case report and review of the literature. J Gastrointest Oncol. 2015 Oct;6(5):E77-81. doi: 10.3978/j.issn.2078-6891.2015.048. PMID: 26487955; PMCID: PMC4570907.
- Gupta DTK, Brasfield RD. Metastatic melanoma: a clinicopathologic study. Cancer. 1964; 17:1323-1339.
- LARMI TK. Malignant melanoma of the gallbladder. Report of a case resulting in an external biliary fistula. Acta Chir Scand. 1960 Oct 15; 119:502-5. PMID: 13759132.
- PAUTLER EE, GALLAVAN EM. Melanoma of brain and gallbladder. AMA Arch Pathol. 1951 Feb;51(2):238-45. PMID: 14799042.
- THAYER KH, WILLIAMS OO, ROWE D. Malignant melanoma of the gallbladder; report of a case and review of the literature. Ariz Med. 1955 Jan;12(1):15-8. PMID: 13229757.
- JONES CH. Malignant melanoma of the gall-bladder. J Pathol Bacteriol. 1961 Apr; 81:423-30. doi: 10.1002/path.1700810215. PMID: 13790499.
- Peison B, Rabin L. Malignant melanoma of the gallbladder: report of three cases and review of the literature. Cancer. 1976 May;37(5):2448-54. doi: 10.1002/1097-0142(197605)37:5<2448::aid-cncr2820370538>3.0.co;2-q. PMID: 1260727.
- Sierra-Callejas JL, Warecka K. Primary malignant melanoma of the gallbladder. Virchows Arch A Pathol Anat Histol. 1976 Jun 22;370(3):233-8. doi: 10.1007/BF00427583. PMID: 821238.
- WALSH TS Jr. Primary melanoma of the gallbladder with cervical metastasis and fourteen and a half year survival; first histologically proved case. Cancer. 1956 May-Jun;9(3):518-22. doi: 10.1002/1097-0142(195605/06)9:3<518::aid-cncr2820090313>3.0.co;2-3. PMID: 13330001.
- McFadden PM, Krementz ET, McKinnon WM, Pararo LL, Ryan RF. Metastatic melanoma of the gallbladder. Cancer. 1979 Nov;44(5):1802-8. doi: 10.1002/1097-0142(197911)44:5<1802::aid-cncr2820440539>3.0.co;2-7. PMID: 387209.
- Antohe M, Coman A, Turcu G, Nedelcu RI, Brinzea A, Balaban M, Moroianu A, Manea L, Hulea I, Balasescu E, Zurac SA, Cioplea M, Popp C, Nichita L, Ion DA. The prognostic significance of the clinical and histological parameters in primary cutaneous melanoma patients. Med Pharm Rep. 2022 Jul;95(3):229-235. doi: 10.15386/mpr-2142. Epub 2022 Jul 26. PMID: 36060503; PMCID: PMC9387583.
- Hussein Al-Janabi M, Mohammad JG, Mohsen AY, Saad A, Issa R. Metastatic melanoma to the gallbladder presented as a polyp with acute cholecystitis: A case report from Syria. Ann Med Surg (Lond). 2022 Mar 28; 76:103514. doi: 10.1016/j.amsu.2022.103514. PMID: 35495390; PMCID: PMC9052169.
- Doole EL, Gan P, Klein O. A rare case of solitary gallbladder metastasis from an early cutaneous melanoma. Clin Case Rep. 2021 Oct 18;9(10):e04908. doi: 10.1002/ccr3.4908. PMID: 34703598; PMCID: PMC8521313.
- Higgins CM, Strutton GM. Malignant melanoma of the gall bladder--does primary melanoma exist? Pathology. 1995 Oct;27(4):312-4. doi: 10.1080/00313029500169203. PMID: 8771146.
- D'Urso Vilar GG, Iriarte F, Speisky D, Bregante ML, Quildrian SD. Isolated gallbladder metastasis of melanoma: Case report. Int J Surg Case Rep. 2020; 71:311-314. doi: 10.1016/j.ijscr.2020.04.086. Epub 2020 May 14. PMID: 32485637; PMCID: PMC7264460.
- Katz SC, Bowne WB, Wolchok JD, Busam KJ, Jaques DP, Coit DG. Surgical management of melanoma of the gallbladder: a report of 13 cases and review of the literature. Am J Surg. 2007 Apr;193(4):493-7. doi: 10.1016/j.amjsurg.2006.06.033. PMID: 17368297.
- Khan ZS, Huth J, Kapur P, Huerta S. Indications and recommended approach for surgical intervention of metastatic disease to the gallbladder. World J Surg Oncol. 2010 Sep 10; 8:80. doi: 10.1186/1477-7819-8-80. PMID: 20828420; PMCID: PMC2944133.
- Nelson RS, Lanza F. Malignant melanoma metastatic to the upper gastrointestinal tract: endoscopic and radiologic correlations, form and evolution of lesions, and value of directed biopsy in diagnosis. Gastrointest Endosc. 1978 May;24(4):156-8. doi: 10.1016/s0016-5107(78)73493-0. PMID: 648840.
- Ercolino GR, Guglielmi G, Pazienza L, Urbano F, Palladino D, Simeone A. Gallbladder and small bowel metastasis of regressive melanoma: a case report. BJR Case Rep. 2018 Aug 1;5(1):20180032. doi: 10.1259/bjrcr.20180032. PMID: 31131119; PMCID: PMC6519491.
- Patel D, Sohrawardy S, Sedhai YR, Basnyat S, Daxini A, Basu A, Mehta VR, Mohammed A, Lichtenstein S. Metastatic Cutaneous Melanoma of the Gallbladder. Case Rep Gastrointest Med. 2017; 2017:8532379. doi: 10.1155/2017/8532379. Epub 2017 Jan 30. PMID: 28251000; PMCID: PMC5303833.
- Hess GF, Glatz K, Rothschild SI, Kollmar O, Soysal SD, Boll DT, Droeser RA, Mechera R. Malignant melanoma metastasis in the gallbladder. A case report of an unusual metastatic site. Int J Surg Case Rep. 2020; 75:372-375. doi: 10.1016/j.ijscr.2020.09.116. Epub 2020 Sep 19. PMID: 32980711; PMCID: PMC7522582.