More Information
Submitted: October 03, 2023 | Approved: October 23, 2023 | Published: October 24, 2023
How to cite this article: Gonzalez-Mendibil I, García-Pascual E, Villanueva A, García-Gutiérrez S. Bispectral Index Monitoring: Ability to Detect Deep Sedation during Endoscopy. Ann Clin Gastroenterol Hepatol. 2023; 7: 028-034.
DOI: 10.29328/journal.acgh.1001042
Copyright License: © 2023 Gonzalez-Mendibil I, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Bispectral index; Colonoscopy; Sedation
Bispectral Index Monitoring: Ability to Detect Deep Sedation during Endoscopy
Iratxe Gonzalez-Mendibil1,2* , Eduardo García-Pascual1
, Eduardo García-Pascual1 , Ane Villanueva3,4
, Ane Villanueva3,4 and Susana García-Gutiérrez2,3
and Susana García-Gutiérrez2,3
1Department of Anaesthesiology, Galdakao University Hospital, Galdakao, Bizkaia, Spain
2Faculty of Health Sciences, Department of Medicine, University of Deusto, Avenida de las Universidades, 24, 48007 Bilbao (Bizkaia), Spain
3Research Unit, Galdakao University Hospital, Galdakao, Bizkaia, Spain
4Kronikgune Institute for Health Services Research, Barakaldo, Spain
*Address for Correspondence: Iratxe Gonzalez-Mendibil, Department of Anaesthesiology, Galdakao University Hospital, Labeaga Auzoa, 48960, Galdakao, Spain, Email: [email protected]
Background: Clinical practice guidelines recommend monitoring the depth of anesthesia during endoscopic examination of the gastrointestinal tract using sedation scales, despite their subjective nature, while the use of the bispectral index, an objective measure, during sedation, remains controversial. The main objective of this study was to assess the ability of bispectral index monitoring to characterize the depth of anesthesia during endoscopy.
Methods: We conducted a cross-sectional study to assess the performance of the bispectral index using data from a multicentre clinical trial with 180 patients undergoing scheduled colonoscopies. Sedation was monitored using the bispectral index and Ramsay Sedation Scale. Data on sedation were recorded at five-time points (t1 to t5) during the colonoscopy.
Results: Bispectral values were significantly associated with Ramsay scores (rho, -0.73; p < 0.0001). In regression analysis, each unit increase in bispectral value was associated with a reduction in the risk of a high Ramsay score (> 3) at all points (OR 0.922; 95% CI: 0.865–0.979; p < 0.0001 at t1). Receiver operating characteristic curve analysis found areas under the curve of 0.8272 for a bispectral index cut-off for deep sedation of 69.76 (sensitivity, 95.35%; negative predictive value, 97.53%) when reaching the colic flexure (t2) and 0.8399 for a cut-off of 69.29 (sensitivity, 96.15%; negative predictive value, 98.81%) at the end of the colonoscopy (t5).
Conclusion: Bispectral index monitoring enables objective real-time reliable assessment of sedation. It enables easy continuous monitoring with a very good performance for detecting deep sedation and correlates with a clinical scale routinely used in endoscopic procedures.
In recent decades, the development of advanced diagnostic techniques and colorectal cancer screening programs has led to a steady increase in the number of gastrointestinal procedures worldwide. In this context, deep sedation and anaesthetist-led analgesia have become important, enabling better conditions for performing examinations, with a low rate of adverse events and good levels of patient satisfaction [1-4].
Monitoring of hypnosis enables the objective assessment of the depth of anesthesia, making it possible to tailor the anesthesia to each patient. Indeed, the efficacy of this approach has been well demonstrated for general anesthesia [5]. Nonetheless, given the technical and methodological characteristics of these monitoring systems, the results may be less reliable during sedation, thus hindering the generalization of their use.
The 2018 guidelines of the European Society of Anaesthesiology on the management of sedation and analgesia in adults [2] indicated the need for continuous clinical observation, this being the basic level of clinical monitoring required during and after any procedural sedation (very good consensus: level of evidence B, grade of recommendation strong [2]). For this, the depth of sedation must be regularly assessed using one of the validated scales for assessing response to verbal and tactile stimuli [6,7]. It was also concluded that processed electroencephalography monitoring could be considered in sedated patients (very good consensus: level of evidence B, grade of recommendation weak [2]), though its role remains controversial.
The main objective of this study was to assess the ability of a BIS monitoring system to characterize the depth of anesthesia in patients under deep sedation. The evidence available in this context is limited, so we propose the study with the largest sample size published to date investigating different and relevant time points during the colonoscopy.
We designed a cross-sectional diagnostic accuracy study comparing a BIS monitoring system to the Ramsay Sedation Scale as a reference standard. We recruited patients undergoing scheduled colonoscopies under sedation and analgesia. The clinical research ethics committee of Galdakao Hospital (Bizkaia, Spain) evaluated the research project and approved the protocol on 18 January 2018 (protocol 01/18). This study is based on data from a clinical trial. It was conducted in accordance with the Declaration of Helsinki [8] and in compliance with data protection regulations, ensuring that personal data was handled in such a way that no data collected could not be associated with identified or identifiable individuals (Spanish Organic Law 15/1999, 13-12, Personal Data Protection). The trial was registered on ClinicalTrials.gov (identifier NCT03453359). All participants provided written informed consent before their inclusion.
The target population was adult patients undergoing scheduled colonoscopies in Galdakao Hospital (Bizkaia, Spain) or Medaro Hospital (Gipuzkoa, Spain). The inclusion criteria were: age between 18 and 85 years, indication for scheduled colonoscopy, American Society of Anesthesiologists (ASA) physical status class I, II, or III, body mass index under 35 kg/m2, and no neurological dysfunction. We excluded patients with allergies to the drugs used, those with neurological conditions, moderate-to-severe kidney or liver failure, moderate-to-severe lung disease, and long-term opioid users. Sedation was carried out by one of five anesthetists involved in the study, all of whom had extensive experience in this field.
The primary outcome of this study was depth of sedation. We also recorded data on patient characteristics including age, sex, body weight and height, ASA class, potentially relevant comorbidities at baseline, and procedure duration and medication administered.
All patients were monitored noninvasively (Monitor Infinity® Delta, Dräger Medical Systems Inc, USA), following the recommendations in sedation guidelines of international societies [1,2,4]. In addition, all patients were assessed using both the RSS (Table 1) and a BIS monitoring system (BISTM Quatro sensor, for the BISTM VISTA monitor, Aspect Medical System). Notably, the BIS values were recorded separately from the RSS scores, the former by a trained observer and the latter by the anesthetist.
| Table 1: Ramsay Sedation Scale (Adapted from: [7]). | |
| Score | Patient response |
| 1 | Awake; anxious, agitated, or restless, or both |
| 2 | Awake; cooperative, oriented, and tranquil |
| 3 | Awake; patient responds to commands only |
| 4 | Asleep; patient exhibits brisk response to light glabellar tap or loud auditory stimuli |
| 5 | Asleep; patient exhibits sluggish response to light glabellar tap or loud auditory stimuli |
| 6 | Asleep; patient exhibits no response to stimuli |
Both RSS scores and BIS values were recorded at five-time points during the procedure: t1, at the start of the colonoscopy, t2, when the endoscope reached the right colic flexure; t3, at the start of endoscope removal; t4, during resection of the first polyp, and t5, at the end of the procedure. We accepted BIS readings provided that they had a signal quality index > 50 and were obtained from electroencephalographic traces with no electromyogenic artifacts.
Total intravenous sedation was provided using a computer-controlled infusion system with an AlarisTM PK syringe pump, following the Marsh model for propofol, with plasma concentrations between 1 and 3 µg/ml. Remifentanil was administered by continuous infusion with an Alaris TIVA syringe pump at doses between 0.05 and 0.15 µg/kg/min, except in the case of patients who were over 70 years old or ASA class III, for whom the initial delivery rate was reduced to 0.02 µg/kg/min. The target level of sedation was a BIS value of 66 to 75 in the BIS group and an RSS score of 3 to 5 in the controls.
In a previous observational study at Galdakao Hospital, 60% of patients had BIS index values of between 66 and 75, and with long, complex procedures, sedation tends to be deeper. We estimated that we would need at least 83 patients per group to detect an around 20% difference in the percentage of patients under deep sedation, and specifically, to test the main hypothesis that in the control group, the percentage would be 70% while in the BIS group, this percentage would be at least 20% higher. Assuming a loss to follow-up of 20%, we therefore needed 90 patients per group to achieve a level of significance (alpha) of 5% and statistical power (beta) of 80%. These calculations were performed using nQuery Advisor version 7.0.
Qualitative variables were expressed as frequencies and percentages and continuous variables as means and standard deviations. Percentages were compared using the chi-squared test (or Fisher’s exact test, when the expected frequencies were less than 5), and differences between the means for continuous variables were examined using Student’s t-tests or the nonparametric Wilcoxon test, depending on the type of distribution. Correlations between BIS and RSS were assessed using Pearson’s correlation coefficient and the influence of the BIS on having an RSS score > 3, which is the cut-off point that distinguishes deep sedation from mild-to-moderate sedation, was analyzed using a simple logistic regression model at each of the five-time points during the intervention. The predictive performance of each of the models was assessed using the area under the receiver operating characteristic curve. The threshold for statistical significance was set at p < 0.05. All the statistical analysis was carried out using SAS V9.4 (SAS Institute, Inc., Carey, NC).
By the end of 2018, a total of 206 patients who underwent sedation for scheduled colonoscopy had been recruited but 26 were excluded for not meeting the selection criteria. We were left with 180 patients for inclusion in the analysis, reaching the required sample size. Data concerning the demographic and clinical characteristics of patients in each group and the interventions they received are summarised in Table 2.
| Table 2: Descriptive analysis of patient characteristics. | |
| Total | |
| N (%) | |
| Total | 180 |
| Age (years)* | 59.43 (12.57) |
| Body weight (kg)* | 75.90 (13.40) |
| Body mass index (kg/m2)* | |
| <18 | 1 (0.56) |
| 18-25 | 52 (28.89) |
| 25-30 | 93 (51.67) |
| 30-35 | 34 (18.89) |
| Sex (woman) | 74 (41.11) |
| ASA class | |
| I | 34 (18.89) |
| II | 110 (61.11) |
| III | 36 (20.00) |
| Comorbidity† | 146 (81.11) |
| Duration of the procedure (min)* | 26.83 (12.76) |
| Propofol (mg)* | 189.58 (83.28) |
| Remifentanil (µg)* | 135.70 (57.30) |
| N: Number; %: Percentage; *Results expressed as mean (standard deviation). ASA: American Society of Anesthesiologists; †Diagnosis of relevant comorbid conditions including hypertension, diabetes, high cholesterol, inflammatory bowel disease, mild asthma, mild chronic obstructive pulmonary disease, hyperthyroidism, gastritis, and mild kidney disease. | |
A total of 829 BIS value and RSS score measurements were obtained. Overall, 109 patients underwent polyp resection (60.56% of all the patients included). A Pearson’s correlation test yielded an r - value of 0.73 (Table 3), indicating a strong correlation between RSS scores and BIS values.
| Table 3: Correlation between the bispectral index and Ramsay Sedation Scale over the course of the colonoscopy. | ||
| Ramsay Sedation Scale score Pearson’s correlation |
p - value | |
| Bispectral index | ||
| Start of colonoscopy, t1 | -0.7623 | < 0.0001 |
| Endoscope advanced to right colic flexure, t2 | -0.7607 | < 0.0001 |
| Start of endoscope removal, t3 | -0.6791 | < 0.0001 |
| Polyp resection, t4 | -0.6917 | < 0.0001 |
| End of colonoscopy, t5 | -0.7522 | < 0.0001 |
| Mean | -0.7364 | < 0.0001 |
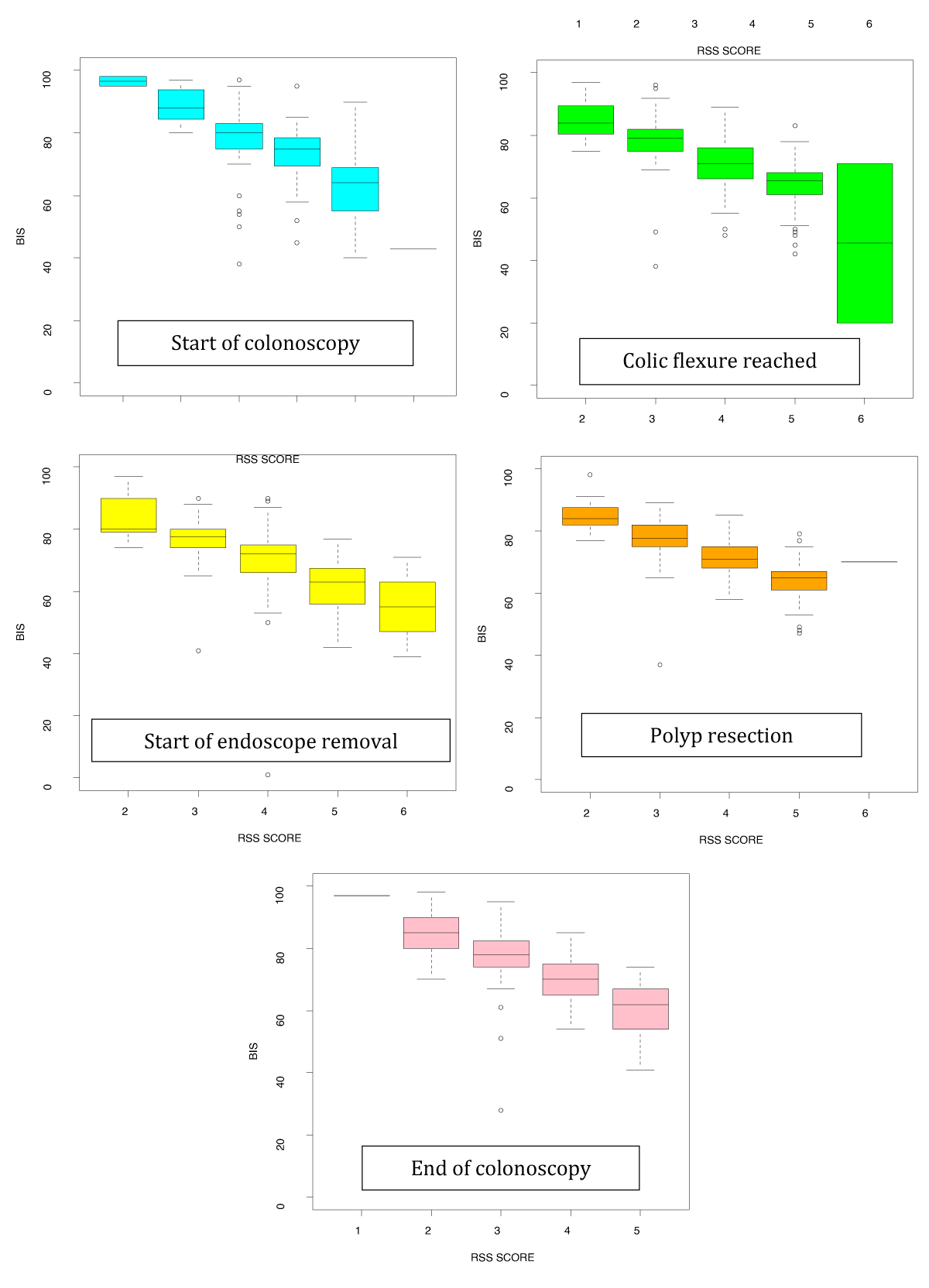
We established that BIS values differed significantly between each level of sedation as assessed by the RSS score. Specifically, lower BIS values were associated with higher RSS scores, that is, deeper sedation, as shown in Figure 1. For each unit increase in BIS value, the probability of being assigned the highest RSS score decreased: by 12.37% at t1 (95% CI 1.149-1.333), 13.31% at t2 (95% CI 1.196-1.481), 12.97% at t3 (95% CI 1.193-1.410), 18.31% at t4 (95% CI 1.371-2.444) and 13.49% at t5 (95% CI 1.193-1.526) (Table 4).
Figure 1: Relationship between bispectral (BIS) values and Ramsay Sedation Scale (RSS) scores. Box diagram of the BIS values obtained at the five time points during the colonoscopy by RSS score.
| Table 4: Results of the logistic regression assessing changes in BIS index reflecting changes in RSS score during the colonoscopy (t1 to t5). | |||
| β (SE) | Odds ratio (95% CI) | p - value | |
| Start of colonoscopy, t1 | |||
| Intercept | 13.55 (2.62) | < 0.0001 | |
| BIS* | -0.21 (0.04) | 0.808 (0.750 – 0.870) | < 0.0001 |
| AUC (95% CI) | 0.922 (0.865 – 0.979) | ||
| Right colic flexure reached, t2 | |||
| Intercept | 18.35 (3.62) | < 0.0001 | |
| BIS* | -0.29 (0.05) | 0.751 (0.675 – 0.836) | < 0.0001 |
| AUC (95% CI) | 0.916 (0.864 – 0.968) | ||
| Start of endoscope removal, t3 | |||
| Intercept | 16.82 (2.82) | < 0.0001 | |
| BIS* | -0.26 (0.04) | 0.771 (0.709 – 0.838) | < 0.0001 |
| AUC (95% CI) | 0.914 (0.866 – 0.962) | ||
| Polyp reception, t4 | |||
| Intercept | 39.05 (9.60) | < 0.0001 | |
| BIS* | -0.61 (0.15) | 0.546 (0.409 – 0.729) | < 0.0001 |
| AUC (95% CI) | 0.940 (0.899 – 0.981) | ||
| End of colonoscopy, t5 | |||
| Intercept | 18.75 (4.17) | < 0.0001 | |
| BIS* | -0.30 (0.06) | 0.741 (0.655 – 0.838) | < 0.0001 |
| AUC (95% CI) | 0.923 (0.873 – 0.973) | ||
| β (SE): Beta Coefficient (standard error); CI: Confidence Interval; AUC: Area Under the Receiver Operating Characteristic Curve. *Estimate for each unit increase | |||
The cut-off values of the BIS index for detecting deep sedation were 69.76 (sensitivity, 95.35%; negative predictive value, 97.53%) at t2, and 69.29 (sensitivity, 96.15%; negative predictive value, 98.81%) at t5 (Tables 5 and 6).
| Table 5: Bispectral index cut-off values for deep sedation as assessed by the Ramsay Sedation Scale score > 3 (vs. ≤ 3) at each of the five measurement time points during the colonoscopy. | ||
| BIS cut-off value | Optimal AUC / Corrected AUC | |
| Colonoscopy | ||
| Start of colonoscopy | 72.11 | 0.8779 / 0.8622 |
| Right colic flexure reached | 69.76 | 0.8490 / 0.8272 |
| Start of endoscopy removal | 64.11 | 0.8598 / 0.8343 |
| Polyp resection | 67.33 | 0.8595 / 0.8311 |
| End of colonoscopy | 69.29 | 0.8606 / 0.8399 |
| AUC: Area Under the Receiver Operating Characteristic Curve | ||
| Table 6: Procedure-specific validity estimates for the bispectral (BIS) monitoring system for predicting deep sedation. | ||||
| Ramsay Sedation Scale score | Total | Estimate (95% CI) | ||
| t1: Start of colonoscopy | ||||
| BIS | > 3 | ≤ 3 | Sn: 93.94% (85.80%-100%) | |
| ≤ 75 | 31 | 33 | 64 | Sp: 77.55% (70.81%-84.30%) |
| > 75 | 2 | 114 | 116 | PPV: 48.44% (36.19%-60.68%) |
| Total | 33 | 147 | 180 | NPV: 98.28% (95.91%-100%) |
| t2: Right colic flexure reached | ||||
| BIS | > 3 | ≤ 3 | Sn: 95.35% (89.05%-100%) | |
| ≤ 75 | 41 | 58 | 99 | Sp: 57.66% (49.39%-65.94%) |
| > 75 | 2 | 79 | 81 | PPV: 41.41% (31.71%-51.12%) |
| Total | 43 | 137 | 180 | NPV: 97.53% (94.15%-100%) |
| t3: Start of endoscope removal | ||||
| BIS | > 3 | ≤ 3 | Sn: 96.30% (91.26%-100%) | |
| ≤ 75 | 52 | 57 | 109 | Sp: 54.76% (46.07%-63.45%) |
| > 75 | 2 | 69 | 71 | PPV: 47.71% (38.33%-57.08%) |
| Total | 54 | 126 | 180 | NPV: 97.18% (93.33%-100%) |
| t4: Polyp resection | ||||
| BIS | > 3 | ≤ 3 | Sn: 100% (100%-100%) | |
| ≤ 75 | 27 | 46 | 73 | Sp: 43.90% (33.16%-54.64%) |
| >75 | 0 | 36 | 36 | PPV: 36.99% (25.91%-48.06%) |
| Total | 27 | 82 | 109 | NPV: 100% (100%-100%) |
| t5: End of colonoscopy | ||||
| BIS | > 3 | ≤ 3 | Sn: 96.15% (88.76%-100%) | |
| ≤ 75 | 25 | 71 | 96 | Sp: 53.90% (46.02%-61.77%) |
| >75 | 1 | 83 | 84 | PPV: 26.05% (17.26%-34.82%) |
| Total | 26 | 154 | 180 | NPV: 98.81% (94.49%-100%) |
| Sn: sensitivity; Sp: Specificity; PPV: Positive Predictive Value; NPV: Negative Predictive Value. | ||||
The results of this study indicate that the BIS index performs very well in identifying patients with deep sedation, allowing us to assess the level of sedation objectively, reliably, and in real-time, without interrupting the procedure. They demonstrate that BIS readings are correlated with RSS scores during sedation using propofol and remifentanil. Further, this study determines cut-off BIS values indicative of deep sedation in scheduled colonoscopies, with high predictive accuracy.
Several studies have concluded that there is no evidence to support the routine use of the BIS index in endoscopic procedures [9-13]. Nonetheless, motivated by the goal of improving patient safety and optimizing the care provided to patients under deep sedation, we believe that the use of this system for monitoring deep sedation should be considered, especially when using propofol [2]. BIS monitoring minimizes complications, is associated with a high level of satisfaction among patients and endoscopists [14], and enables more effective titration with a corresponding reduction in the duration of sedation [15-17], the use of such a monitoring system is particularly advisable in the case of complex examinations [1].
In endoscopic sedation, previous research has indicated that the BIS index is strongly correlated with sedation scales, of which the RSS is one of the most widely used [15,18,19]. This association has also been observed in other clinical settings, including intensive care [20-23], palliative sedation [24], and pediatric analgesia [25]. Nonetheless, few studies have focused on the BIS values obtained in endoscopic sedation, values that might enable us to predict and minimize adverse cardiopulmonary events. Bower, et al. [26] and Yu, et al. [27] defined optimal BIS cut-off values of 82 and 91, respectively, for maintaining moderate sedation; but did not define cut-offs for deep sedation. Qadeer, et al. in 2008 [9] published the results from elective ambulatory endoscopic procedures, showing BIS monitoring to have poor sensitivity and accuracy for detecting deep sedation, but the study had some limitations. More recently, cut-off points have been established for respiratory depression under deep sedation; however, the findings cannot be generalized due to the small sample size [28]. Our study focuses on one of the procedures most commonly used for the diagnosis and treatment of gastrointestinal disorders. Every year, 13 to 15 million colonoscopies are performed in the United States of America [29] and more than 540,000 in the United Kingdom [30], in part due to colorectal cancer screening programs and population aging. Thus, the identification of deep sedation with this monitoring, established in our study at BIS values between 64 and 72, allows us to detect patients at risk of airway obstruction and respiratory depression.
The strengths of our study include the results being analyzed for five specific time points during a colonoscopy, and to our knowledge, this study is the first to conduct this type of analysis. Previous authors have defined the parameters obtained every 30 seconds to 3 minutes [9,11,15,26]; however, such regular intervals are not linked to the stage of the procedure or requirement for sedation in given patients. Further, frequent stimulation to assess sedation level might lead to the administration of more medication and higher sedation scores, without increasing the validity of the results. We believe that assessing the findings as a function of the stage of the endoscopic procedure allows us to obtain more relevant and practical information, potentially useful for optimizing the level of sedation and hence improving the satisfaction of both patients and health professionals involved in sedation and endoscopic procedures. Our results suggest that BIS monitoring could reduce the incidence and severity of respiratory events during scheduled colonoscopies by early identifying patients most susceptible to these events. This would allow clinicians to take appropriate preventive measures in each case.
Nonetheless, our study also has some limitations. First, the population analyzed only included ASA class I to III patients who underwent elective endoscopic procedures; that is, we did not assess more complex patients, who might have had different BIS values. This exclusion of complex cases may restrict the external validity of the findings, though it allowed us to obtain conclusive data for the population studied. A second limitation is related to the recording of the depth of the anesthesia. While BIS monitoring provides a continuous objective measure of this level, the interpretation of monitoring based on the RSS may vary with the observer carrying out the measurement. Nevertheless, this scale has been validated, it is widely recommended by working groups, and its use has become widespread in daily clinical practice. Further, the same small group of anesthetists and observers collected all the data for the study, reducing the impact of inter-observer variability.
Strengths and limitations of this study
This study determines cut-off BIS values indicative of deep sedation in scheduled colonoscopies.
This study evaluates BIS values obtained at the five time points during the colonoscopy, in which the stimulus varies considerably, and correlates them with the RSS score.
One limitation is that patients with multiple pathologies were not evaluated.
Our findings indicate that BIS monitoring provides an accurate reliable measurement of the level of sedation, with very good performance for detecting deep sedation in scheduled endoscopic procedures.
Footnotes
Availability of data: Data are available on request from the authors.
Ethics approval: This study involves human participants and was approved by the Ethics Committee of Galdakao Hospital (Bizkaia, Spain) approved the protocol on 18th January 2018 (protocol 01/18). This study is based on data from a clinical trial registered on ClinicalTrials.gov (identifier NCT03453359).
Funding statement: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
- Igea F, Casellas JA, González-Huix F, Gómez-Oliva C, Baudet JS, Cacho G, Simón MA, De la Morena E, Lucendo A, Vida F; Spanish Society of Digestive Endoscopy. Sedation for gastrointestinal endoscopy. Endoscopy. 2014 Aug;46(8):720-31. doi: 10.1055/s-0034-1377561. Epub 2014 Jul 25. PMID: 25061964.
- Hinkelbein J, Lamperti M, Akeson J, Santos J, Costa J, De Robertis E, Longrois D, Novak-Jankovic V, Petrini F, Struys MMRF, Veyckemans F, Fuchs-Buder T, Fitzgerald R. European Society of Anaesthesiology and European Board of Anaesthesiology guidelines for procedural sedation and analgesia in adults. Eur J Anaesthesiol. 2018 Jan;35(1):6-24. doi: 10.1097/EJA.0000000000000683. PMID: 28877145.
- Sidhu R, Turnbull D, Newton M, Thomas-Gibson S, Sanders DS, Hebbar S, Haidry RJ, Smith G, Webster G. Deep sedation and anaesthesia in complex gastrointestinal endoscopy: a joint position statement endorsed by the British Society of Gastroenterology (BSG), Joint Advisory Group (JAG) and Royal College of Anaesthetists (RCoA). Frontline Gastroenterol. 2019 Apr;10(2):141-147. doi: 10.1136/flgastro-2018-101145. Epub 2019 Jan 9. PMID: 31205654; PMCID: PMC6540268.
- Gotoda T, Akamatsu T, Abe S, Shimatani M, Nakai Y, Hatta W, Hosoe N, Miura Y, Miyahara R, Yamaguchi D, Yoshida N, Kawaguchi Y, Fukuda S, Isomoto H, Irisawa A, Iwao Y, Uraoka T, Yokota M, Nakayama T, Fujimoto K, Inoue H. Guidelines for sedation in gastroenterological endoscopy (second edition). Dig Endosc. 2021 Jan;33(1):21-53. doi: 10.1111/den.13882. Epub 2020 Dec 9. PMID: 33124106.
- Johansen JW. Update on bispectral index monitoring. Best Pract Res Clin Anaesthesiol. 2006 Mar;20(1):81-99. doi: 10.1016/j.bpa.2005.08.004. PMID: 16634416.
- Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, Schwam EM, Siegel JL. Validity and reliability of the Observer's Assessment of Alertness/Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol. 1990 Aug;10(4):244-51. PMID: 2286697.
- Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. Br Med J. 1974 Jun 22;2(5920):656-9. doi: 10.1136/bmj.2.5920.656. PMID: 4835444; PMCID: PMC1613102.
- Mellin-Olsen J, Staender S, Whitaker DK, Smith AF. The Helsinki Declaration on Patient Safety in Anaesthesiology. Eur J Anaesthesiol. 2010 Jul;27(7):592-7. doi: 10.1097/EJA.0b013e32833b1adf. PMID: 20520556.
- Qadeer MA, Vargo JJ, Patel S, Dumot JA, Lopez AR, Trolli PA, Conwell DL, Stevens T, Zuccaro G Jr. Bispectral index monitoring of conscious sedation with the combination of meperidine and midazolam during endoscopy. Clin Gastroenterol Hepatol. 2008 Jan;6(1):102-8. doi: 10.1016/j.cgh.2007.10.005. Epub 2007 Dec 11. PMID: 18065278.
- Kang KJ, Min BH, Lee MJ, Lim HS, Kim JY, Lee JH, Chang DK, Kim YH, Rhee PL, Rhee JC, Kim JJ. Efficacy of Bispectral Index Monitoring for Midazolam and Meperidine Induced Sedation during Endoscopic Submucosal Dissection: A Prospective, Randomized Controlled Study. Gut Liver. 2011 Jun;5(2):160-4. doi: 10.5009/gnl.2011.5.2.160. Epub 2011 Jun 24. PMID: 21814595; PMCID: PMC3140660.
- Lera dos Santos ME, Maluf-Filho F, Chaves DM, Matuguma SE, Ide E, Luz Gde O, de Souza TF, Pessorrusso FC, de Moura EG, Sakai P. Deep sedation during gastrointestinal endoscopy: propofol-fentanyl and midazolam-fentanyl regimens. World J Gastroenterol. 2013 Jun 14;19(22):3439-46. doi: 10.3748/wjg.v19.i22.3439. PMID: 23801836; PMCID: PMC3683682.
- Fruchter O, Tirosh M, Carmi U, Rosengarten D, Kramer MR. Prospective randomized trial of bispectral index monitoring of sedation depth during flexible bronchoscopy. Respiration. 2014;87(5):388-93. doi: 10.1159/000358440. Epub 2014 Mar 6. PMID: 24602973.
- Zhang H, Lu Y, Wang L, Lv J, Ma Y, Wang W, Li G, Li Y. Bispectral index monitoring of sedation depth during endoscopy: a meta-analysis with trial sequential analysis of randomized controlled trials. Minerva Anestesiol. 2019 Apr;85(4):412-432. doi: 10.23736/S0375-9393.18.13227-5. Epub 2019 Jan 4. PMID: 30621373.
- Imagawa A, Fujiki S, Kawahara Y, Matsushita H, Ota S, Tomoda T, Morito Y, Sakakihara I, Fujimoto T, Taira A, Tsugeno H, Kawano S, Yagi S, Takenaka R. Satisfaction with bispectral index monitoring of propofol-mediated sedation during endoscopic submucosal dissection: a prospective, randomized study. Endoscopy. 2008 Nov;40(11):905-9. doi: 10.1055/s-2008-1077641. PMID: 19023932.
- Bell JK, Laasch HU, Wilbraham L, England RE, Morris JA, Martin DF. Bispectral index monitoring for conscious sedation in intervention: better, safer, faster. Clin Radiol. 2004 Dec;59(12):1106-13. doi: 10.1016/j.crad.2004.04.008. PMID: 15556593.
- Park SW, Lee H, Ahn H. Bispectral Index Versus Standard Monitoring in Sedation for Endoscopic Procedures: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2016 Mar;61(3):814-24. doi: 10.1007/s10620-015-3945-9. Epub 2015 Nov 3. PMID: 26531839.
- Lin YJ, Wang YC, Huang HH, Huang CH, Liao MX, Lin PL. Target-controlled propofol infusion with or without bispectral index monitoring of sedation during advanced gastrointestinal endoscopy. J Gastroenterol Hepatol. 2020 Jul;35(7):1189-1195. doi: 10.1111/jgh.14943. Epub 2019 Dec 6. PMID: 31802534.
- Yang KS, Habib AS, Lu M, Branch MS, Muir H, Manberg P, Sigl JC, Gan TJ. A prospective evaluation of the incidence of adverse events in nurse-administered moderate sedation guided by sedation scores or Bispectral Index. Anesth Analg. 2014 Jul;119(1):43-48. doi: 10.1213/ANE.0b013e3182a125c3. PMID: 24413547.
- Jokelainen J, Mustonen H, Kylänpää L, Udd M, Lindström O, Pöyhiä R. Assessment of sedation level for endoscopic retrograde cholangiopancreatography - a prospective validation study. Scand J Gastroenterol. 2018 Mar;53(3):370-375. doi: 10.1080/00365521.2018.1435715. Epub 2018 Feb 7. PMID: 29411681.
- Mondello E, Panasiti R, Siliotti R, Floridia D, David A, Trimarchi G. BIS and Ramsay score in critically ill patient: what future? Minerva Anestesiol. 2002 Jan-Feb;68(1-2):37-43. PMID: 11877559.
- Riess ML, Graefe UA, Goeters C, Van Aken H, Bone HG. Sedation assessment in critically ill patients with bispectral index. Eur J Anaesthesiol. 2002 Jan;19(1):18-22. doi: 10.1017/s0265021502000030. PMID: 11913799.
- Consales G, Chelazzi C, Rinaldi S, De Gaudio AR. Bispectral Index compared to Ramsay score for sedation monitoring in intensive care units. Minerva Anestesiol. 2006 May;72(5):329-36. English, Italian. PMID: 16675941.
- Hernández-Gancedo C, Pestaña D, Peña N, Royo C, Pérez-Chrzanowska H, Criado A. Monitoring sedation in critically ill patients: bispectral index, Ramsay and observer scales. Eur J Anaesthesiol. 2006 Aug;23(8):649-53. doi: 10.1017/S0265021506000056. Epub 2006 Jan 27. PMID: 16438768.
- Monreal-Carrillo E, Allende-Pérez S, Hui D, García-Salamanca MF, Bruera E, Verástegui E. Bispectral Index monitoring in cancer patients undergoing palliative sedation: a preliminary report. Support Care Cancer. 2017 Oct;25(10):3143-3149. doi: 10.1007/s00520-017-3722-8. Epub 2017 Apr 29. PMID: 28456907.
- Agrawal D, Feldman HA, Krauss B, Waltzman ML. Bispectral index monitoring quantifies depth of sedation during emergency department procedural sedation and analgesia in children. Ann Emerg Med. 2004 Feb;43(2):247-55. doi: 10.1016/s0196-0644(03)00721-2. PMID: 14747816.
- Bower AL, Ripepi A, Dilger J, Boparai N, Brody FJ, Ponsky JL. Bispectral index monitoring of sedation during endoscopy. Gastrointest Endosc. 2000 Aug;52(2):192-6. doi: 10.1067/mge.2000.107284. PMID: 10922090.
- Yu YH, Han DS, Kim HS, Kim EK, Eun CS, Yoo KS, Shin WJ, Ryu S. Efficacy of bispectral index monitoring during balanced propofol sedation for colonoscopy: a prospective, randomized controlled trial. Dig Dis Sci. 2013 Dec;58(12):3576-83. doi: 10.1007/s10620-013-2833-4. Epub 2013 Aug 28. PMID: 23982208.
- Kilic ET, Sayar S. Can Bispectral Index Monitoring (EEG) be an Early Predictor of Respiratory Depression under Deep Sedation during Endoscopic Retrograde Cholangiopancreatography? Sisli Etfal Hastan Tip Bul. 2020 Dec 11;54(4):444-450. doi: 10.14744/SEMB.2020.10476. PMID: 33364885; PMCID: PMC7751237.
- Peery AF, Crockett SD, Murphy CC, Jensen ET, Kim HP, Egberg MD, Lund JL, Moon AM, Pate V, Barnes EL, Schlusser CL, Baron TH, Shaheen NJ, Sandler RS. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2021. Gastroenterology. 2022 Feb;162(2):621-644. doi: 10.1053/j.gastro.2021.10.017. Epub 2021 Oct 19. PMID: 34678215.
- Ravindran S, Thomas-Gibson S, Bano M, Robinson E, Jenkins A, Marshall S, Ashrafian H, Darzi A, Coleman M, Healey C. National census of UK endoscopy services 2021. Frontline Gastroenterol. 2022 May 9;13(6):463-470. doi: 10.1136/flgastro-2022-102157. PMID: 36250173; PMCID: PMC9555135.