More Information
Submitted: September 04, 2023 | Approved: September 11, 2023 | Published: September 12, 2023
How to cite this article: Abdelgader MAE, Ibrahim AS, Abbas SE, Suliman AA, Ahmed MSI, et al. Pattern of Clinical Presentation and Management of Inflammatory Bowel Disease. Ann Clin Gastroenterol Hepatol. 2023; 7: 011-018.
DOI: 10.29328/journal.acgh.1001040
Copyright License: © 2023 Abdelgader MAE, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Pattern; Clinical; Presentation; Management; Inflammatory bowel; Disease
Pattern of Clinical Presentation and Management of Inflammatory Bowel Disease
Mohamed Abdalla Elamin Abdelgader1, Abdelgadir Suliman Ibrahim1, Sara Elfadel Abbas2, Awadalla Abdelwahid Suliman3*, Mohamed Suliman I Ahmed1, Safa Mohamed Ibrahim4 and Abdelmoneim Altayeb Abdo1
1Consultant Physician, MD of Internal Medicine Sudan, Sudan Medical Specialization Board (SMSB), Sudan
2Consultant Gastroenterologist, Soba University Hospital, Sudan
3Assistant Professor of Obstetrics and Gynecology, Faculty of Medicine, Al Neelain University, Sudan
4Consultant Physician, Emirate Health Services, Umlquwain Hospital, UAE
*Address for Correspondence: Awadalla Abdelwahid Suliman, Assistant professor of Obstetrics and Gynecology, Faculty of Medicine, Al Neelain University, Khartoum, Sudan, Email: [email protected]
Background: Inflammatory bowel disease (IBD) is characterized by non-specific chronic relapsing inflammation of the gastrointestinal tract and extra-intestinal manifestations. It includes Crohn’s disease (CD) ulcerative colitis (UC) and unclassified colitis.
Objective: To assess the clinical presentations and management of inflammatory bowel disease in Sudanese patients.
Methodology: Prospective, cross-section hospital-based study was conducted at Soba University Hospital (SUH) and Ibn Sina Hospital, in a period from December 2016 to March 2017.
Data was entered and analyzed with SPSS, an interview questionnaire containing demographic, clinical, type of IBD, treatment, and complications.
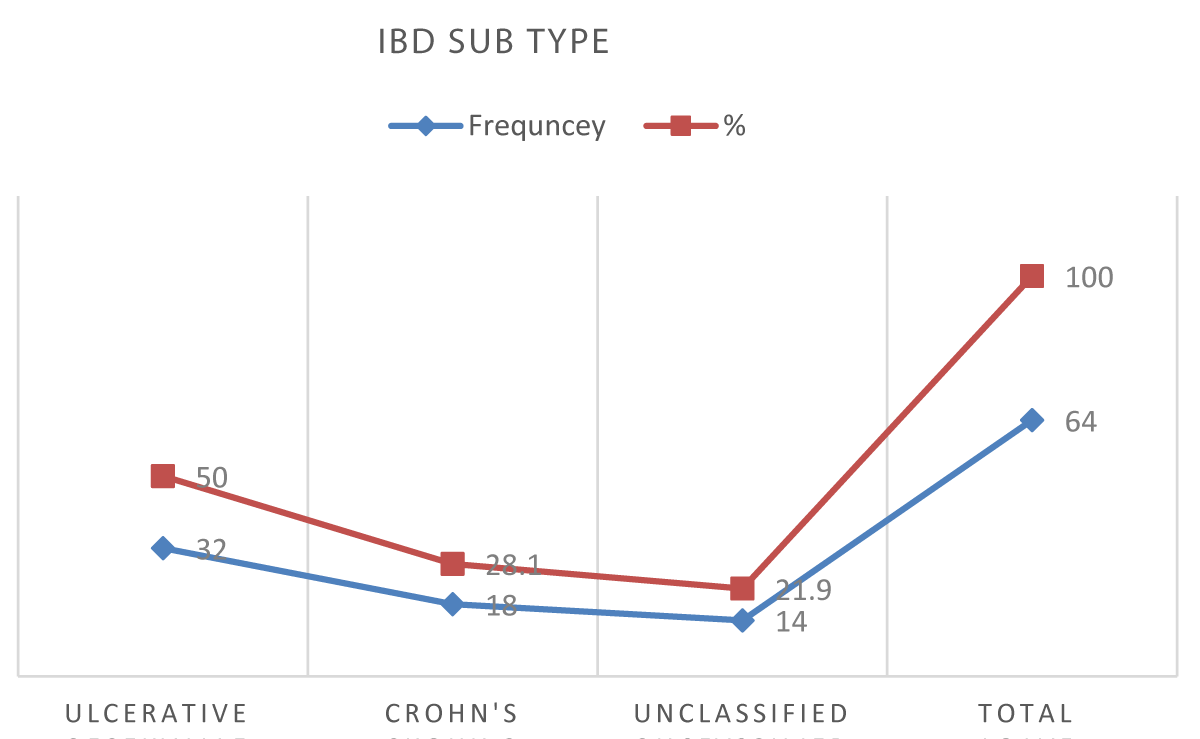
Results: A total of 64 IBD patients were included, 50% were diagnosed with UC, 28.1% with CD and 21.9% unclassified type.
The most frequent age in UC patients was 41 – 50 years 34.4%, in CD was 31- 40 years 38.9% and for the unclassified type was 51 – 70 years 57.2%.
The female was higher in CD while males were higher in Ulcerative colitis disease, symptoms were diarrhea, rectal bleeding, abdominal pain, rectal pain, tenesmus and fatigue.
Study participants received 5 amino salicylic acid, and steroids, especially in the oral formulation. Minimal usage of topical forms, azathioprine, and biological agents.
Conclusion: The study concluded UC is more common than CD. This should be taken into account as an important update for internal medicine professionals to adjust their expectations and lines of diagnosis, and management. The emergence of the unclassified type in Sudan requires good communication between the pathologists and the physicians and MDT meetings in every patient with suspicion of IBD.
Inflammatory bowel disease (IBD) is characterized by non-specific chronic relapsing inflammation of the gastrointestinal tract and extra-intestinal manifestations. The two main disease categories are Crohn’s disease (CD) and ulcerative colitis (UC), which have both distinct and overlapping clinical and pathological features [1-3].
There is also a third type which is unclassified colitis occurs in patients who have clinical and endoscopic evidence of chronic IBD affecting the colon without small bowel involvement and no definite histological or other finding suggesting either CD or UC [4,5].
The prevalence of CD appears to be higher in urban areas than in rural areas, and also in high socio-economic classes [6], but the incidence of IBD is now rising in developing countries and is increasingly considered an emerging global disease [7].
Ulcerative colitis and Crohn’s disease share many extraintestinal manifestations, although some of these tend to occur more commonly with either condition. Eye-skin-mouth-joint extraintestinal manifestations (e.g., oral aphthae, erythema nodosum, large-joint arthritis, and episcleritis) reflect active disease, whereas pyoderma gangrenosum, primary sclerosing cholangitis (PSC), ankylosing spondylitis, uveitis, kidney stones, and gallstones may occur in quiescent disease [8,9].
Systemic symptoms are common in IBD and include fever, sweats, malaise, and arthralgia [10,11]. The rectum is always involved in ulcerative colitis, and the disease primarily involves continuous lesions of the mucosa and the submucosa. Both ulcerative colitis and Crohn’s disease usually have waxing and waning intensity and severity. When the patient is symptomatic due to active inflammation, the disease is considered to be in an active stage (the patient is having a flare of the IBD) [12].
It was a descriptive, prospective, Hospital-based study conducted in a period from December 2016 to March 2017 at Soba University Hospital (SUH) and IbnSina Hospital which were referral hospitals from other States. Study populations included all patients diagnosed with Inflammatory Bowel Disease at IbnSina Hospital and Soba Hospital. The inclusion criteria age above 18 years old and any patient diagnosed with inflammatory bowel disease within the study period. The study excluded ages below 18 years old and other co-morbidities (chronic renal failure, chronic liver failure, and malignancies).
There were 64 patients included in this study. Data collection tools are used to collect different information. With direct Face-to-face questionnaire interviews at the referring clinic. Patients diagnosed with IBD were included after a written informed consent was taken, clinical presentation ascertained, the diagnosis based on colonoscopy and histopathology then the type of management received was written. All the recruited patients were under follow-up throughout the study period. The investigator and research assistants were included.
Every patient enrolled in this study had undergone a general physical examination. Study socio-demographic variables were age, gender, education level, residence, and occupation and dependent variables were clinical presentation, endoscopy findings, and histopathological test.
Data analysis was done using SPSS version 22.0 descriptive statistics in terms of frequency tables with percentages and graphs, bi-variable analysis to determine the associations between the main outcome variable and the other relevant factors with the Chi-square test (for categorical variables), p value of 0.05 or less is considered statistically significant, data represented after analysis in form of uni-variable tables, cross tabulation (bi variable tables), figures and narrative illustration.
Ethical considerations by written ethical clearance and approval for conducting this research was obtained from Sudan Medical Specialization Board Ethical Committee, written ethical clearance was obtained from Khartoum state MOH, written permission was obtained from the administrative authority of Soba and IbnSina hospitals, written informed consent was taken individually from all participants and study data/information was used for the research purposes only. The privacy issues were intentionally considered.
This is a cross-sectional study covering 64 patients who were diagnosed with inflammatory bowel disease at Soba and IbnSina hospitals in 2017, 50% were diagnosed with UC, 28.1% with CD and 21.9% remained unclassified. The mean age in UC was 43.3, while it was 34.3 in CD and 47.4 in the unclassified type (Figure 1).
Figure 1: IBD subtypes in the study participants (n = 64).
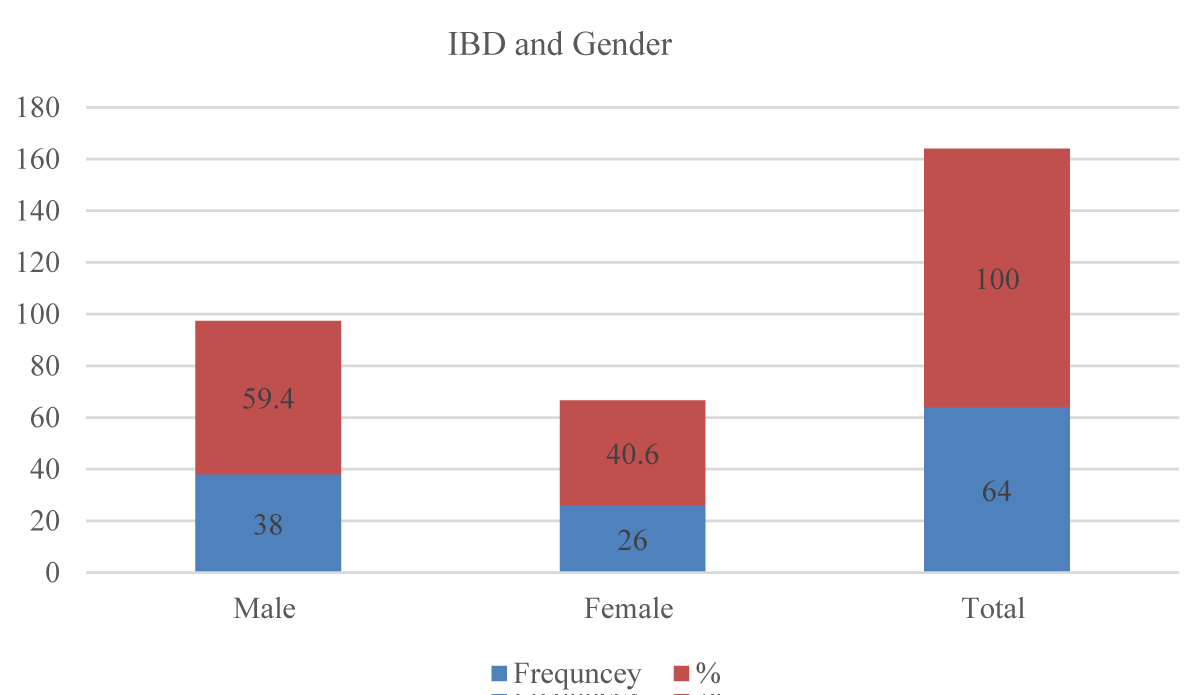
Regarding gender, the overall male-to-female ratio was (1.46:1), 38 (59.4%) of all the study participants were males while the females were 26(40.6%) (Figure 2).
Figure 2: IBD subtypes and gender in the study participants (n = 64).
According to IBD subtypes, the males to female ratio in UC was (1.9:1), males were 21 (65.6%) while the females were 11(34.4%), In CD the males to female ratio was (1:1.4), males were 7 (38.9%) while the females were 11(61.1%), In the Unclassified type the males to female’s ratio was (2.5:1), male were10 (71.4%) while the females were 4 (28.6%).
Regarding the residences, 35(54.7%) of the study participants live in urban areas while 29(45.3%) of them live in rural areas and the majority of our study participants were nonsmokers 93.8% while the smokers were 4 (6.2%).
The most frequent symptom was diarrhea in 55 (85.9%) of overall study participants, according to IBD subtypes, 32(100%) of UC patients, 11(61.1%) of CD patients, and 12 (85.7%) of unclassified type patients have diarrhea.
The most frequent symptoms in UC were diarrhea in 32 (100%), rectal bleeding in 28 (87.5%), abdominal pain in 21 (65.6%), rectal pain in 18 (56.2%) and tenesmus in 18 (56.2%). While the most frequent symptoms in CD were abdominal pain in 17 (94.4%) and diarrhea in 11 (61.1%), in the unclassified type the diarrhea was in 12 (85.7%), followed by rectal bleeding in 11 (78.6), abdominal pain in 11 (71.4%) and rectal pain in 6 (42.9).
Fatigability was noted in 12 (66.7%) of CD patients, while it occurred in 11(34.4%) of UC patients and in 4 (28.6%) of the unclassified type patients (Table 1).
| Table 1: Symptoms according to IBD subtypes among the study participants (n = 64). | ||||||||||||
| IBD subtype | Diarrhea | Rectal bleeding | Rectal pain | Tenesmus | Abdominal pain | Fatigue | ||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Ulcerative | 32 | 100 | 28 | 87.5 | 18 | 56.2 | 18 | 56.2 | 21 | 65.6 | 11 | 34.4 |
| Crohn's | 11 | 61.5 | 1 | 5.6 | 0 | 0 | 1 | 5.6 | 17 | 94.4 | 12 | 66.7 |
| Unclassified | 12 | 85.7 | 11 | 78.6 | 6 | 42.9 | 3 | 21.4 | 10 | 71.4 | 4 | 28.6 |
| Total | 55 | 85.9 | 40 | 62.5 | 24 | 37.5 | 22 | 34.4 | 48 | 75 | 27 | 42.2 |
| P - value | .001 | < .001 | < 0.001 | .001 | 0.073 | .043 | ||||||
| Chi square | 14.417 | 34.980 | 15.771 | 14.455 | 5.225 | 6.287 | ||||||
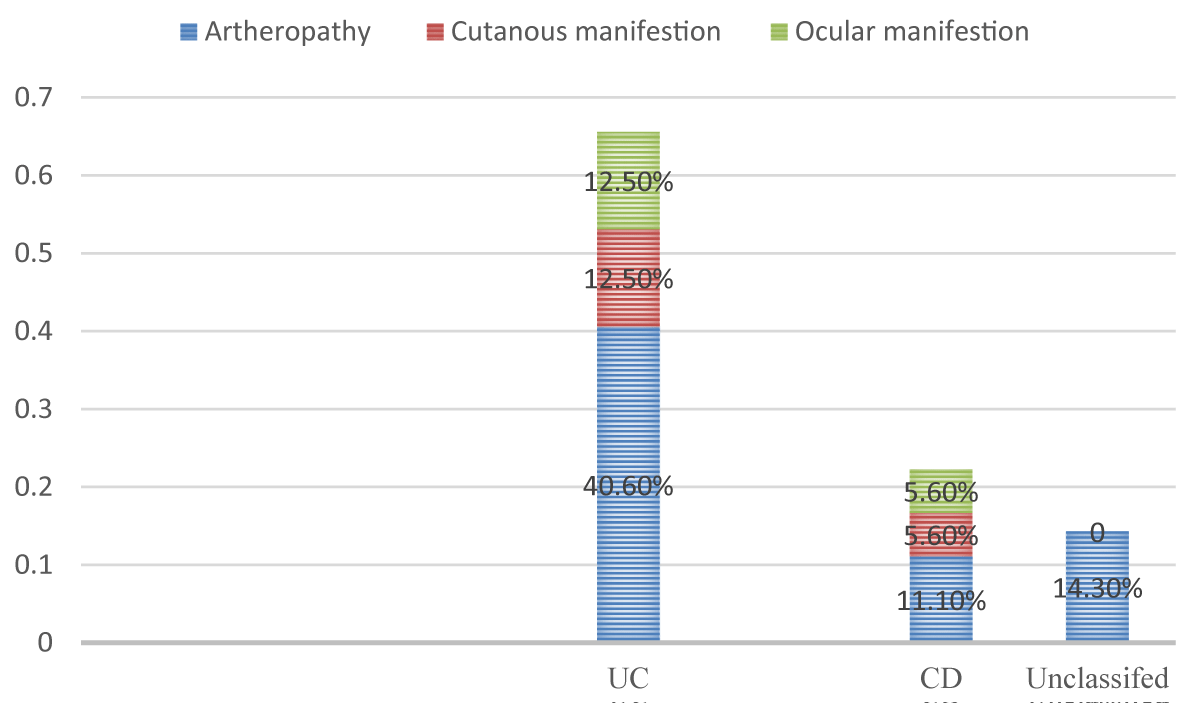
The distribution of extra intestinal manifestations among the study participants were as follows, In UC were arthropathy in 13(40.6%), cutaneous manifestations in 4 (12.5%), and ocular manifestations in 4 (12.5%). In CD were arthropathy in 2 (11.1%), cutaneous manifestations in 1(5.6%), and ocular manifestations in 1(5.6%). In the unclassified type, there was just 2 (14.3%) had arthropathy (Figure 3).
Figure 3: Extra-intestinal manifestations of IBD subtypes (n = 64).
Regarding the occurrence of complications, the perianal fistula occurred in 1 (3.1%) patient with UC and 2(11.1%) patients with CD. While the abdominal abscess occurred in 1 (3.1%) patient with UC. There were no complications in the study participants with the unclassified type disease.
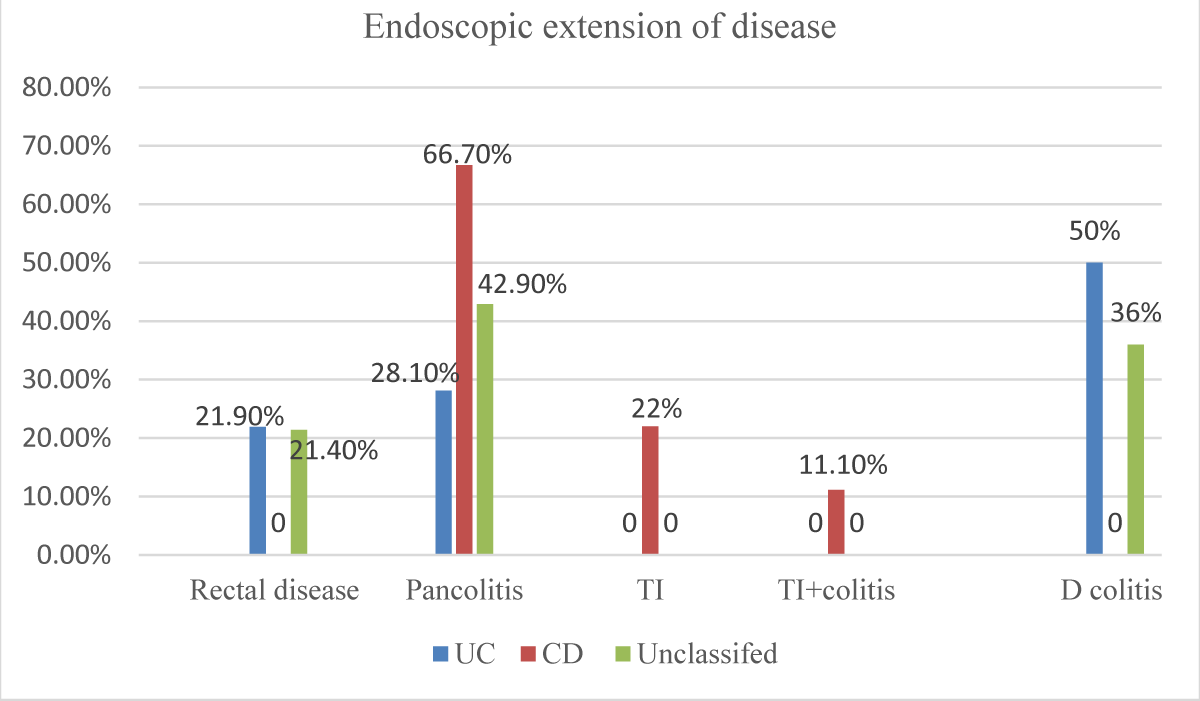
With regard to the endoscopic extension of the disease at index colonoscopy, in UC they were distributed as Pancolitis in 9 (28.1%), distal colitis in 16(50%), and proctitis in7 (21.9%), while in CD there were Pancolitis in 12 (66.7%), terminal ileum in 4(22.2%) and 2 (11.1%) in terminal ileum +colitis disease and in the unclassified type there were Pancolitis in 6 (42.9%), distal colitis in 5 (35.7%) and proctitis in 3 (21.4%). No patient within the study group had perianal disease. All of the IBD study participants received medical treatment, while there were 3(4.7) underwent surgical operations (Figure 4).
Figure 4: Endoscopic extension of disease at index colonoscopy among IBD subtypes in the study participants (n = 64).
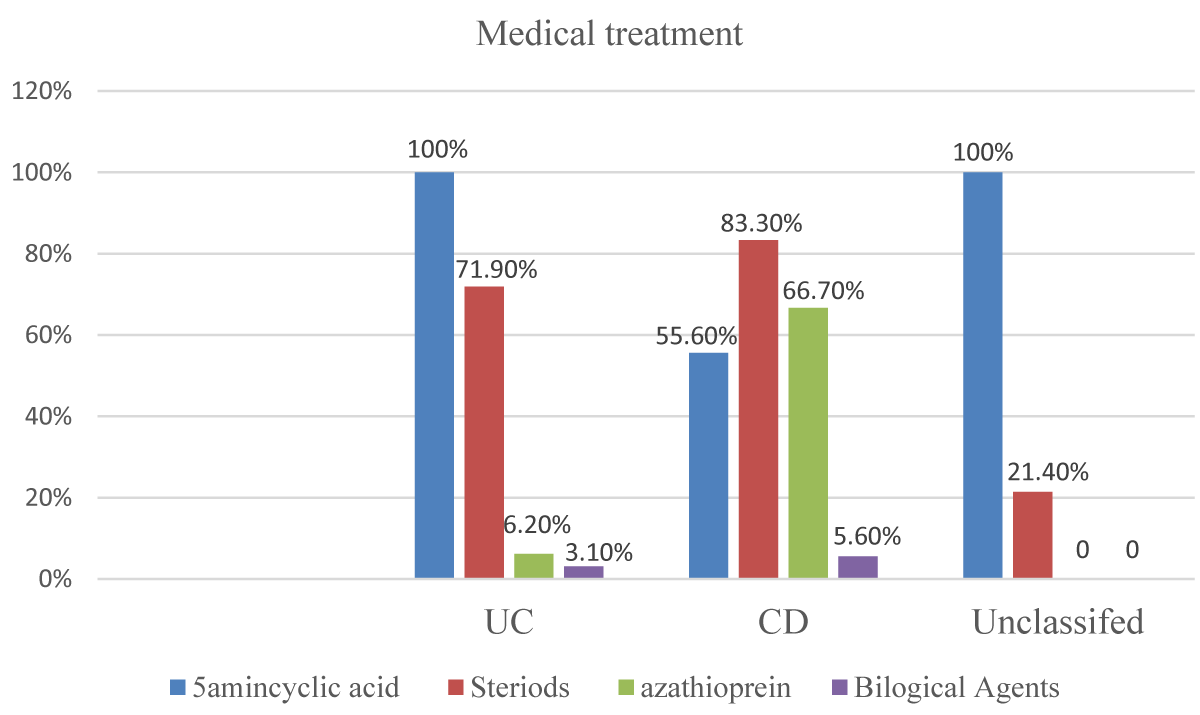
Medical treatment received, the patients with UC received 5 aminsalicylic acids were 32(100%), steroid were 23 (71.9%), azathioprine was 2 (6.2%) and biological agent were 1(3.1%), while patients with CD received 5 aminsalicylic acids were 10(55.6%), steroid was 16 (88.95), azathioprine was 12 (66.7%) and biological agent was 1 (5.6%) and patients with unclassified type received 5 aminosalicylic acids were 14 (100%) and steroid were 3 (21.4%) (Figure 5).
Figure 5: Medical management received by the study participants according to IBD subtypes (n = 64).
Study participants who received oral 5 amino salicylic acids were 54(84.4%), according to IBD subtypes they were 31(96.9%) in UC patients,10(55.6%) in CD patients and13(92.9%) in unclassified type patients. While the study participants received topical 5 aminsalicylic acid were 4(6.2%), they were distributed into 3 (9.4%) of UC patients and 1 (7.1%) of unclassified type patients. Table 2. Study participants who received oral steroids were 41 (64.1%), according to IBD subtype they were 23(71.9%) in UC patients, 15(83. 3%) in CD patients, and 3 (21.4%) in unclassified type patients, intra venous steroid were received by 3(4.7%) of the IBD study participants, they are 2 (6.2%) patients with UC and 1 patient with CD (Table 2).
| Table 2: Medical treatment according to IBD subtypes among the study participants (n = 64). | ||||
| IBD subtype | 5aminosalicylic acid | Steroids | ||
| N | % | N | % | |
| Ulcerative | 32 | 100 | 23 | 71.9 |
| Crohn's | 10 | 55.6 | 16 | 88.9 |
| Unclassified | 14 | 100 | 3 | 21.4 |
| Total | 56 | 87.5 | 42 | 65.6 |
| P-value | .000 | < .001 | ||
| Chi-square | 23.365a | 16.995 | ||
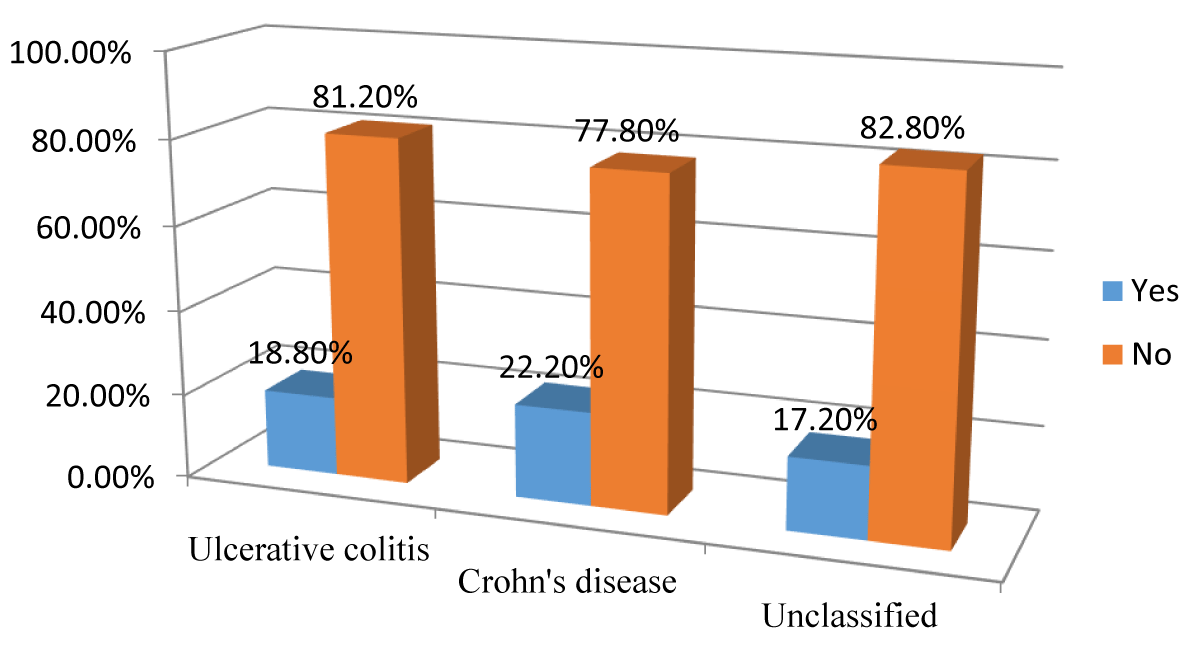
The flare-up during the last year occurred in 11 (17.2%) patients of the study participants, they were 6 (18.8%) patients of UC patients 4 (22.2%) CD patients, and 1(7.1%) of the unclassified type patients (Figure 6).
Figure 6: The incidence of Flare-ups during the Last Year according to IBD subtypes among the study participants (n = 64).
This is a cross-sectional study that covered 64 patients who were diagnosed with inflammatory bowel disease recruited during the study period at Soba and IbnSina hospitals in 2017.
Half of the study participants 50% were diagnosed with ulcerative colitis, 18 (28.1%) with Crohn’s disease, and 14 (21.9%) diagnosed as unclassified type.UC is more common than CD, this is like most of the world studies [13-15], This is also like Ibrahim M.'s study concerning Inflammatory Bowel Disease in Sudanese Patients: in 2011 [16], We also keeping with him in having a significant number of patients diagnosed with the unclassified type, this could be due to the lack of effective communication between clinicians and pathologists and the lack of effective clinical data that enable the pathologists to make a clear diagnosis.
This study found that the most frequent age group in ulcerative colitis patients was 41 – 50 years in 11(34.4%), in Crohn’s disease was 31- 40 years in 7 (38.9%), and for the unclassified type was 51 – 70 years 8 (57.2%), this is almost in keeping with Ibrahim M. study [16]. Within the context of the participant’s age, a study of Inflammatory Bowel Disease: An Expanding Global Health Problem by M'Koma AE [17] claimed that IBD is now affecting a much younger population presents an additional concern. Meta-analyses conducted on patients acquiring IBD at a young age also reveal a trend for their increased risk of developing colorectal cancer (CRC) [17].
Our study found the overall male-to-female ratio was (1.46:1), 38 (59.4%) of all the study participants were males while the females were 26(40.6%). According to IBD subtypes, the males are more than the females in UC, while in CD the females are more than the males. These results are like some world studies [18,19] but unlike the Egyptian study by Esmat S, et al. [9], the researchers found that the males were less frequent than females in UC and the reverse for CD.
Our study found that there is a significant relation between the residence and the IBD subtype (p = 0.01). Urbanization was more associated with UC while rural residency was more found with CD, which is like Ibrahim M.'s study [12], in that seventy percent of his cases were residing in central relatively more urbanized areas of the country. The urban population were 54.7% compared with rural 45.3% this variation is explained by migration to urban in recent years where several factors may be involved in these increased risks, including population density, education, lifestyle changes, and potentially, exposure to industrial agents, exposure to SO2 and NO2 may increase the risk of early onset UC and CD, respectively, these data lend support to the hypothesis that components of industrialization, such as pollution, may play a role in the development and course of IBD. These findings further argue that factors associated with an urban lifestyle influence one’s risk of IBD. It is unclear whether the relationship occurs due to the environment itself or in combination with one’s genetic predisposition to the disease [20].
Living in an urban setting has been associated with an increased risk for IBD through a series of studies conducted in the last six decades. In a systematic review published in 2012, living in an urban setting was associated with an increased risk of both UC and CD [21].
Smoking among study populations, the majority of our study participants were nonsmokers, 93.8% while the smokers were 6.2%, which is the most widely and longest studied environmental exposure associated with IBD. To date, it has been observed that smoking has a varying impact on CD and UC, contributing to an increased risk for individuals with CD and a protective role in individuals with UC.
Thirteen studies examined the relationship between UC and smoking, whereas 9 examined the relationship between CD and smoking. We found evidence of an association between current smoking and CD (OR, 1.76; 95% confidence interval [CI], 1.40-2.22) and former smoking and UC (OR, 1.79; 95% CI, 1.37-2.34). Current smoking had a protective effect on the development of UC when compared with controls (OR, 0.58; 95% CI, 0.45-0.75) [22].
In this study the most frequent symptom was diarrhea in 55 (85.9%) of overall study participants, with a higher occurrence among UC than CD, there was significant variation with diarrhea (p = 0.01), which is like most of the world studies [23,24].
The most frequent symptoms in overall study participants were diarrhea, rectal bleeding, abdominal pain, rectal pain in and tenesmus this is like a study in Libya by Ahmaida AI et about Inflammatory Bowel Disease in Libya: Epidemiological and Clinical features [23].
The distribution of extra intestinal manifestations among the study participants were as follows, In UC were arthropathy in 13(40.6%), cutaneous manifestations in 4 (12.5%), and ocular manifestations in 4 (12.5%). In CD were arthropathy in 2 (11.1%), cutaneous manifestations in 1(5.6%), and ocular manifestations in 1(5.6%). In the unclassified type, there was just 2 (14.3%) had arthropathy, while the complications, the perianal fistula occurred in 1 (3.1%) patient with UC and 2(11.1%) patients with CD, and the abdominal abscess occurred in 1 (3.1%) patient with UC. There were no complications in the study participants with the unclassified type disease. The minimal occurrence of extra intestinal manifestations and complications is probably due to the relatively few number of study participants which is similar to [9].
Regarding the endoscopic extent of the disease at the index colonoscopy, this study found that Pancolitis was the most common type, indicating that IBD presented in the most severe form in our study participants, and there was a significant variation with Pancolitis (p = 0.03).
In this study the terminal ileum disease occurred exclusively in CD in 4(22.2%), p-value <.05, this signifies the importance of reaching and carefully visualizing the ileum during an endoscopic procedure. Our study found that there was no significant variation in perianal, rectal, or terminal ileum with colitis with IBD subtypes (p values > 0.05), while there was a significant variation with distal colitis (p < 0.05), These results are like the study of inflammatory bowel disease in India--changing paradigms. By Ray G1 [25], the researchers found that Sixty-five percent of their UC patients presented with Pancolitis, and the majority had severe clinical, endoscopic, and histological disease, like a study done in China by Xia B, et al. [24,26,27].
In this study all the participants received medical treatment, while Three Three (4.7%) underwent surgical operations, the medical treatment received, patients with UC received 5 aminsalicylic acids 32(100%), steroids were 23 (71.9%), azathioprine was 2 (6.2%)and the biological agent was 1(3.1%), while patients with CD received 5 aminsalicylic acids were 10(55.6%), the steroid was 16 (88.95), azathioprine was 12 (66.7%) and biological agent were 1 (5.6%) and patients with unclassified type received 5 aminosalicylic acids were 14 (100%) and steroid were 3 (21.4%).
Unlike most of the IBD treatment guidelines which recommended steroids for acute disease and flare up only [28-30], there was relatively excessive usage of steroids among our study participants 42(65.6%) p - value (> 0.05), this is most probably due to unavailability and expensive price of immune modifier drugs and the biological agents in Sudan, while the steroid are readily available. Regarding the use of azathioprine, the study found a significant variation according to IBD subtypes (p < 0.05) but not with the use of biological agents (p < 0.669). Immunosuppressants, such as azathioprine, that require several weeks to achieve their therapeutic effect have a limited role in the acute setting but are preferred for long-term management [31]. Immunosuppressant drugs can be an invaluable adjunct therapy for the treatment of patients with intractable inflammatory bowel disease or complex, inoperable perianal disease. Although immunosuppressant agents have significant side effects, they are safer and better tolerated than long-term corticosteroid therapy. Should not be used in young patients who are candidates for surgery or in patients who are non-compliant and refuse to return for periodic monitoring [32].
In this study, participants received oral 5 aminosalicylic acids were 54(84.4%), While the study participants received topical 5 aminsalicylic acids were 4(6.2%). Of the Study participants who received oral steroid 41 (64.1%), while the intra venous steroid was received by 3(4.7%) of the IBD study participants. Although the topical form of 5 aminosalicylic and steroid importance was proved [28], they were minimally used by our study participants, this is likely because they are expensive and not covered by insurance companies. 5-ASA is the active therapeutic moiety, absorbed in the upper gastrointestinal tract, the efficacy of 5-ASA preparations (e.g., sulfasalazine) in CD is less striking, with a modest benefit at best in controlled trials [33].
Although not statistically significant (p – value > 0.05), most of the study participants' disease remained quiescent during the last year, and the flare-up during the last year occurred in 11 (17.2%) patients of the study participants.
When we go through our findings with a similar Sudanese context in Sudan, the research of SE Khalifa, et al. [34], we have agreement and conflict, the majority of their patients with UC improved with medical treatment. They concluded that IBD is not a rare disease in Sudan, with UC being more common than CD. The disease tends to be more common in men in both UC and CD.
Corticosteroids are highly effective induction agents [35,36] but maintenance studies have demonstrated that this effect is not durable [35-37]. Additionally, they have well-documented side effects, such as increased infection risk, avascular bone necrosis, mood disturbance, hypothalamic–pituitary–adrenal axis suppression, osteoporosis, Cushingoid appearance, and hypertension [38].
Study participants who received oral steroids were 41 (64.1%), according to the IBD subtype they were 35.9% in UC patients, 23. 3% in CD patients and 4.7% in unclassified type patients, while the intra venous steroids were received by 4.7% of the IBD study participants, they are 3.1% of patients with UC, and 1 patient with CD, in contrast with the study [39], which CD patients were more likely than UC patients to have disease judged as moderate/severely active at the time of clinic visit (23.8% vs. 17.6%.While steroids remain the cornerstone of inducing remission in IBD, their longer-term or repeated use is discouraged because of a lack of long-term efficacy and an unacceptable level of side effects. Avoiding excess steroid use is therefore a key aim for clinicians and patients [40,41].
The rapid induction and maintenance of remission are the main principles of treatment for UC. Corticosteroids, 5-aminosalicylates (5-ASA), immunomodulators, biologics, and small molecules are the foundation of UC treatment. The most cost-effective immunomodulators, such as azathioprine (AZA), have been used to treat UC for many decades, primarily to maintain remission [42].
This study had some limitations. The relatively few number of study participants only 64 may affect negatively the probability of finding significant relationships between different factors or variables with the IBD subtypes. Another limitation, some outcomes may need to be followed over more time. So, a cohort follow-up design may be useful for a more detailed description of the outcome among IBD patients. Also, this study was conducted in two centers so, the results may not be generalized, highlighting the need for a multicenter trial with the uniform assessment protocol.
The study found that the most frequent symptoms in overall study participants were diarrhea, rectal bleeding, abdominal pain, rectal pain, tenesmus, and fatigue. Also, the study found that pancreatitis was the most common type at the index colonoscopy. Most of the study participants received 5 aminosalicylic acids, and steroids, especially in the oral formulation but there is minimal usage of topical forms, also there is minimal use of azathioprine, and biological agents. There is age variation in IBD subtypes and higher proportions of females in Crohn’s disease more males in Ulcerative colitis disease.
The unavailability and the expensive price of immune modifier drugs and biological agents in Sudan should be urgently addressed and further studies are recommended with a larger sample size for a more in-depth investigation of relevant factors and patient characteristics and treatment effectiveness.
- Ukwenya AY, Ahmed A, Odigie VI, Mohammed A. Inflammatory bowel disease in Nigerians: still a rare diagnosis? Ann Afr Med. 2011 Apr-Jun;10(2):175-9. doi: 10.4103/1596-3519.82067. PMID: 21691027.
- Xia B, Crusius J, Meuwissen S, Pe?a A. Inflammatory bowel disease: definition, epidemiology, etiologic aspects, and immunogenetic studies. World J Gastroenterol. 1998 Oct;4(5):446-458. doi: 10.3748/wjg.v4.i5.446. PMID: 11819343; PMCID: PMC4767749.
- Verstockt B, Bressler B, Martinez-Lozano H, McGovern D, Silverberg MS. Time to Revisit Disease Classification in Inflammatory Bowel Disease: Is the Current Classification of Inflammatory Bowel Disease Good Enough for Optimal Clinical Management? Gastroenterology. 2022 Apr;162(5):1370-1382. doi: 10.1053/j.gastro.2021.12.246. Epub 2022 Jan 4. PMID: 34995534.
- Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006 Jun;55(6):749-53. doi: 10.1136/gut.2005.082909. PMID: 16698746; PMCID: PMC1856208.
- Ramos GP, Papadakis KA. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin Proc. 2019 Jan;94(1):155-165. doi: 10.1016/j.mayocp.2018.09.013. PMID: 30611442; PMCID: PMC6386158.
- Farrokhyar F, Swarbrick ET, Irvine EJ. A critical review of epidemiological studies in inflammatory bowel disease. Scand J Gastroenterol. 2001 Jan;36(1):2-15. doi: 10.1080/00365520150218002. Erratum in: Scand J Gastroenterol 2001 Jun;36(6):facing 561. PMID: 11218235.
- Bernstein CN, Shanahan F. Disorders of a modern lifestyle: reconciling the epidemiology of inflammatory bowel diseases. Gut. 2008 Sep;57(9):1185-91. doi: 10.1136/gut.2007.122143. Epub 2008 May 30. PMID: 18515412.
- Agrawal D, Rukkannagari S, Kethu S. Pathogenesis and clinical approach to extraintestinal manifestations of inflammatory bowel disease. Minerva Gastroenterol Dietol. 2007 Sep;53(3):233-48. PMID: 17912186.
- Oh SJ, Choi Y, Kim CR, Park JH, Lee JH, Lee DY. An Atypical Extraintestinal Manifestation in a Child with Ulcerative Colitis: Cutaneous Leukocytoclastic Vasculitis. Ann Dermatol. 2020 Feb;32(1):90-91. doi: 10.5021/ad.2020.32.1.90. Epub 2019 Dec 27. PMID: 33911718; PMCID: PMC7992630.
- Harbord M, Annese V, Vavricka SR, Allez M, Barreiro-de Acosta M, Boberg KM, Burisch J, De Vos M, De Vries AM, Dick AD, Juillerat P, Karlsen TH, Koutroubakis I, Lakatos PL, Orchard T, Papay P, Raine T, Reinshagen M, Thaci D, Tilg H, Carbonnel F; European Crohn’s and Colitis Organisation. The First European Evidence-based Consensus on Extra-intestinal Manifestations in Inflammatory Bowel Disease. J Crohns Colitis. 2016 Mar;10(3):239-54. doi: 10.1093/ecco-jcc/jjv213. Epub 2015 Nov 27. PMID: 26614685; PMCID: PMC4957476.
- Yu YR, Rodriguez JR. Clinical presentation of Crohn's, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin Pediatr Surg. 2017 Dec;26(6):349-355. doi: 10.1053/j.sempedsurg.2017.10.003. Epub 2017 Oct 5. PMID: 29126502.
- Conrad K, Roggenbuck D, Laass MW. Diagnosis and classification of ulcerative colitis. Autoimmun Rev. 2014 Apr-May;13(4-5):463-6. doi: 10.1016/j.autrev.2014.01.028. Epub 2014 Jan 11. PMID: 24424198.
- Esmat S, El Nady M, Elfekki M, Elsherif Y, Naga M. Epidemiological and clinical characteristics of inflammatory bowel diseases in Cairo, Egypt. World J Gastroenterol. 2014 Jan 21;20(3):814-21. doi: 10.3748/wjg.v20.i3.814. PMID: 24574754; PMCID: PMC3921490.
- Kappelman MD, Rifas-Shiman SL, Kleinman K, Ollendorf D, Bousvaros A, Grand RJ, Finkelstein JA. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007 Dec;5(12):1424-9. doi: 10.1016/j.cgh.2007.07.012. Epub 2007 Sep 29. PMID: 17904915.
- Hein R, Köster I, Bollschweiler E, Schubert I. Prevalence of inflammatory bowel disease: estimates for 2010 and trends in Germany from a large insurance-based regional cohort. Scand J Gastroenterol. 2014 Nov;49(11):1325-35. doi: 10.3109/00365521.2014.962605. Epub 2014 Sep 26. PMID: 25259808.
- Ibrahim M. Inflammatory Bowel Disease in Sudanese Patients: A Pathological Study (Doctoral dissertation, UOFK).
- M'Koma AE. Inflammatory bowel disease: an expanding global health problem. Clin Med Insights Gastroenterol. 2013 Aug 14;6:33-47. doi: 10.4137/CGast.S12731. PMID: 24833941; PMCID: PMC4020403.
- Moum B, Vatn MH, Ekbom A, Fausa O, Aadland E, Lygren I, Sauar J, Schulz T. Incidence of inflammatory bowel disease in southeastern Norway: evaluation of methods after 1 year of registration. Southeastern Norway IBD Study Group of Gastroenterologists. Digestion. 1995;56(5):377-81. doi: 10.1159/000201262. Erratum in: Digestion 1996;57(2):104. PMID: 8549880.
- Gower-Rousseau C, Vasseur F, Fumery M, Savoye G, Salleron J, Dauchet L, Turck D, Cortot A, Peyrin-Biroulet L, Colombel JF. Epidemiology of inflammatory bowel diseases: new insights from a French population-based registry (EPIMAD). Dig Liver Dis. 2013 Feb;45(2):89-94. doi: 10.1016/j.dld.2012.09.005. Epub 2012 Oct 27. PMID: 23107487.
- Ponder A, Long MD. A clinical review of recent findings in the epidemiology of inflammatory bowel disease. Clin Epidemiol. 2013 Jul 25;5:237-47. doi: 10.2147/CLEP.S33961. PMID: 23922506; PMCID: PMC3728155.
- Soon IS, Molodecky NA, Rabi DM, Ghali WA, Barkema HW, Kaplan GG. The relationship between urban environment and the inflammatory bowel diseases: a systematic review and meta-analysis. BMC Gastroenterol. 2012 May 24;12:51. doi: 10.1186/1471-230X-12-51. PMID: 22624994; PMCID: PMC3517531.
- Mahid SS, Minor KS, Soto RE, Hornung CA, Galandiuk S. Smoking and inflammatory bowel disease: a meta-analysis. Mayo Clin Proc. 2006 Nov;81(11):1462-71. doi: 10.4065/81.11.1462. Erratum in: Mayo Clin Proc. 2007 Jul;82(7):890. PMID: 17120402.
- Ahmaida A, Al-Shaikhi S. Childhood Inflammatory Bowel Disease in Libya: Epidemiological and Clinical features. Libyan J Med. 2009 Jun 1;4(2):70-4. doi: 10.4176/081210. PMID: 21483512; PMCID: PMC3066718.
- Xia B, Shivananda S, Zhang GS, Yi JY, Crusius J, Peka AS. Inflammatory bowel disease in the Hubei Province of China. World J Gastroenterol. 1997 Jun 15;3(2):119-20. doi: 10.3748/wjg.v3.i2.119. PMID: 27041967; PMCID: PMC4801915.
- Ray G. Inflammatory bowel disease in India--changing paradigms. Int J Colorectal Dis. 2011 May;26(5):635-44. doi: 10.1007/s00384-010-1084-5. Epub 2010 Nov 10. PMID: 21063715.
- Mohammed Vashist N, Samaan M, Mosli MH, Parker CE, MacDonald JK, Nelson SA, Zou GY, Feagan BG, Khanna R, Jairath V. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst Rev. 2018 Jan 16;1(1):CD011450. doi: 10.1002/14651858.CD011450.pub2. PMID: 29338066; PMCID: PMC6491285.
- Chen H, Wu L, Wang M, Shao B, Ye L, Zhang Y, Cao Q. Use of the ulcerative colitis endoscopic index of severity and Mayo endoscopic score for predicting the therapeutic effect of mesalazine in patients with ulcerative colitis. Laparosc. Endosc Robot Surg. 2021; 4: 33-39.
- Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L, Lees C, Ho GT, Satsangi J, Bloom S; IBD Section of the British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011 May;60(5):571-607. doi: 10.1136/gut.2010.224154. PMID: 21464096.
- Spinelli A, Bonovas S, Burisch J, Kucharzik T, Adamina M, Annese V, Bachmann O, Bettenworth D, Chaparro M, Czuber-Dochan W, Eder P, Ellul P, Fidalgo C, Fiorino G, Gionchetti P, Gisbert JP, Gordon H, Hedin C, Holubar S, Iacucci M, Karmiris K, Katsanos K, Kopylov U, Lakatos PL, Lytras T, Lyutakov I, Noor N, Pellino G, Piovani D, Savarino E, Selvaggi F, Verstockt B, Doherty G, Raine T, Panis Y. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Surgical Treatment. J Crohns Colitis. 2022 Feb 23;16(2):179-189. doi: 10.1093/ecco-jcc/jjab177. PMID: 34635910.
- Alsoud D, Verstockt B, Fiocchi C, Vermeire S. Breaking the therapeutic ceiling in drug development in ulcerative colitis. Lancet Gastroenterol Hepatol. 2021 Jul;6(7):589-595. doi: 10.1016/S2468-1253(21)00065-0. Epub 2021 May 19. PMID: 34019798.
- Pearson DC, May GR, Fick GH, Sutherland LR. Azathioprine and 6-mercaptopurine in Crohn disease. A meta-analysis. Ann Intern Med. 1995 Jul 15;123(2):132-42. doi: 10.7326/0003-4819-123-2-199507150-00009. PMID: 7778826.
- Meyers S, Sachar DB: Medical therapy of Crohn’s disease. In: Inflammatory Bowel Disease. 4th edn. Eds.Kersnar JB, Shorter RG, Williams and Wilkins, Baltimore. 1995; 695-714.
- Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD). Pharmacol Rep. 2011;63(3):629-42. doi: 10.1016/s1734-1140(11)70575-8. PMID: 21857074.
- Khalifa SE, Mudawi HM, Fedail SS. Presentation and management outcome of inflammatory bowel disease in Sudan. Trop Gastroenterol. 2005 Oct-Dec;26(4):194-6. PMID: 16737049.
- Benchimol EI, Seow CH, Steinhart AH, Griffiths AM. Traditional corticosteroids for induction of remission in Crohn's disease. Cochrane Database Syst Rev. 2008 Apr 16;2008(2):CD006792. doi: 10.1002/14651858.CD006792.pub2. PMID: 18425970; PMCID: PMC6718222.
- Ford AC, Bernstein CN, Khan KJ, Abreu MT, Marshall JK, Talley NJ, Moayyedi P. Glucocorticosteroid therapy in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2011 Apr;106(4):590-9; quiz 600. doi: 10.1038/ajg.2011.70. Epub 2011 Mar 15. PMID: 21407179.
- Steinhart AH, Ewe K, Griffiths AM, Modigliani R, Thomsen OO. Corticosteroids for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2003;(4):CD000301. doi: 10.1002/14651858.CD000301. PMID: 14583917.
- Irving PM, Gearry RB, Sparrow MP, Gibson PR. Review article: appropriate use of corticosteroids in Crohn's disease. Aliment Pharmacol Ther. 2007 Aug 1;26(3):313-29. doi: 10.1111/j.1365-2036.2007.03379.x. Erratum in: Aliment Pharmacol Ther. 2008 Mar 15;27(6):528-9. PMID: 17635367.
- Selinger CP, Parkes GC, Bassi A, Fogden E, Hayee B, Limdi JK, Ludlow H, McLaughlin S, Patel P, Smith M, Raine T. A multi-centre audit of excess steroid use in 1176 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017 Nov;46(10):964-973. doi: 10.1111/apt.14334. Epub 2017 Sep 26. PMID: 28949018.
- Gomollón F, Dignass A, Annese V, Tilg H, Van Assche G, Lindsay JO, Peyrin-Biroulet L, Cullen GJ, Daperno M, Kucharzik T, Rieder F, Almer S, Armuzzi A, Harbord M, Langhorst J, Sans M, Chowers Y, Fiorino G, Juillerat P, Mantzaris GJ, Rizzello F, Vavricka S, Gionchetti P; ECCO. 3rd European Evidence-based Consensus on the Diagnosis and Management of Crohn's Disease 2016: Part 1: Diagnosis and Medical Management. J Crohns Colitis. 2017 Jan;11(1):3-25. doi: 10.1093/ecco-jcc/jjw168. Epub 2016 Sep 22. PMID: 27660341.
- Magro F, Gionchetti P, Eliakim R, Ardizzone S, Armuzzi A, Barreiro-de Acosta M, Burisch J, Gecse KB, Hart AL, Hindryckx P, Langner C, Limdi JK, Pellino G, Zagórowicz E, Raine T, Harbord M, Rieder F; European Crohn’s and Colitis Organisation [ECCO]. Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-anal Pouch Disorders. J Crohns Colitis. 2017 Jun 1;11(6):649-670. doi: 10.1093/ecco-jcc/jjx008. Erratum in: J Crohns Colitis. 2022 Aug 16;: PMID: 28158501. Mallick B, Malik S. Use of Azathioprine in Ulcerative Colitis: A Comprehensive Review. Cureus. 2022 May 10;14(5):e24874. doi: 10.7759/cureus.24874. PMID: 35698683; PMCID: PMC9184176.