More Information
Submitted: December 19, 2022 | Approved: December 30, 2022 | Published: December 31, 2022
How to cite this article: Siddiqui NM, Hari K, Bobat B, Parbhoo D, Lala, et al. Outcome of liver transplantation for autoimmune hepatitis in South Africa. Ann Clin Gastroenterol Hepatol. 2022; 6: 044-050.
DOI: 10.29328/journal.acgh.1001038
Copyright License: © 2022 Siddiqui NM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Black Africans; Autoimmune hepatitis; Liver transplant; Rejection; Recurrence; Outcome
Outcome of liver transplantation for autoimmune hepatitis in South Africa
Nida Mishraz Siddiqui1* , Kapila Hari1, Bilal Bobat1,2, Dinen Parbhoo1,2, Vikash Lala1,3 and Adam Mahomed1-3
, Kapila Hari1, Bilal Bobat1,2, Dinen Parbhoo1,2, Vikash Lala1,3 and Adam Mahomed1-3
1Department of Internal Medicine, Faculty of Health Sciences, University of Witwatersrand, Johannesburg, South Africa
2²Division of Gastroenterology and Hepatology, Wits Donald Gordon Medical Center, South Africa
3Division of Gastroenterology and Hepatology, Charlotte Maxeke Johannesburg Academic Hospital, South Africa
*Address for Correspondence: Nida Mishraz Siddiqui, MBBS, Department of Internal Medicine, Faculty of Health Sciences, University of Witwatersrand, Johannesburg, South Africa, Email: [email protected]
Background: Liver Transplantation (LT) is the definitive treatment for Autoimmune Hepatitis (AIH) in patients with decompensated cirrhosis, liver failure and hepatocellular carcinoma. Outcomes of LT in AIH among black-Africans are not well-defined. We performed a single-center retrospective-review of adult LT patients. The study period was from 1st August 2004-31st August 2019. The primary aim was to document 1- & 5- year patient and graft survival. A secondary aim was to compare the survival of black-Africans to Caucasians. Data was analyzed using survival-analysis.
Results: A total of 56 LT were performed for AIH. Sixty-seven percent (n = 38/56) had confirmed AIH on explant histology. Of these, the majority i.e., 79% (30/38) were female and 21% (8/38) were male. There were equal numbers of black-African 42% (n = 16/38) and Caucasian 42% (n = 16/38) patients. Rejection was four-times higher in black-Africans as compared to Caucasians. Forty-four percent (n = 17/38) had an acute rejection episode and 13% (5/38) had chronic rejection. Recurrence was found in four black-African females. Post-LT patient survival at 1- and 5- years was 86.5% and 80.7%, and graft survival was 94% and 70.8% respectively. The 5- year patient survival was insignificantly lower for black-Africans (73.9%) as compared to Caucasians (83.7%) (p - value 0.26, CI 6.3 - 12.2). Five-year graft survival was significantly lower among black-Africans (55%) as compared to Caucasians (84.8%) (p - value 0.003 CI 3.8 - 8.1)
Conclusion: Black-Africans had a four-fold higher rate of rejection compared to Caucasians. Recurrent AIH was only found in patients of black ethnicity. Similar 1- & 5- year patient survival rates were observed between the two ethnicities. The 5-year graft survival among black-Africans was significantly lower than Caucasians.
Liver Transplantation (LT) is a highly successful mode of treatment for Autoimmune Hepatitis (AIH) with good patient and graft survival rates [1]. Most of the current published data is however, limited to the Caucasian population.
AIH is characterized by progressive, immune mediated destruction of the hepatocytes [2]. It has a female preponderance and occurs in individuals of all ages [3]. The prevalence is 31 cases per 100,000 population [2,3]. AIH represents 4% - 6% of all adult LT in the United States and Europe [1,4,5]. Epidemiological data on the condition is limited for the South African population. In a single tertiary care centre study in South Africa, 11% of patients with AIH required LT [6].
The exact pathogenesis of AIH is unknown, however, genetic predisposition, molecular mimicry and an imbalance between effector and regulatory immunity are key pathogenic components for disease development [7-9]. Recently Human Immunodeficiency Virus (HIV) infection has been implicated as a possible cause of AIH in genetically susceptible individuals. This is an observation based on individual case-reports and reviews [10,11]. Larger studies are needed to support this observation.
The diagnostic International Autoimmune Hepatitis Group (IAIHG) scoring system is validated in the diagnosis of AIH in the pre-transplant setting. Due to the presence of similar antibodies in other autoimmune liver diseases, histology along with characteristic clinical and biochemical findings remains the mode of diagnosis [7].
Patients with treated AIH do not have a normal life expectancy [12]. However, LT for AIH is associated with good outcomes, reaching survival rates at 1- and 5- years of approximately 90% and 80%, respectively. According to a European LT registry, the 5-, 10- and 15-year survival rates of patients after LT for AIH were 79.4%, 70.8% and 60.3% with graft survival rates of 73.2%, 63.4% and 47.3%, respectively [13]. A study from Germany reported a 78.2% 5-year survival rate post LT for AIH [14]. The existing data is largely limited to the Caucasian population, with limited information available on African Americans. There is very little information on the outcome of LT in black-Africans from sub-Saharan Africa.
Our local clinical experience suggests that AIH takes a more aggressive course among black-Africans. It not only presents with more advanced disease but also requires higher doses of immunosuppression to maintain remission. This observation is consistent with findings in African Americans, where they presented with more severe disease and required higher doses of immunosuppression [15]. Their 5- year survival rate was also lower as compared to their Caucasian counterparts [15,16]. Given the paucity of data from South Africa, we conducted a retrospective study to determine the 1- & 5- year patient and graft survival post LT for AIH. A secondary aim was to determine effect of race, if any on the rejection and survival rate.
Study design
This was a single-centre retrospective observational study. The study analysed the 1- and 5- year survival of patients transplanted for AIH. Data included baseline demographics and pre- and post-LT variables such as donor type, explant results, time to recurrence, rejection and failure. Duration of survival was calculated from the date of transplant to the date of event i.e. failure of graft or death of patient. Patients who did not experience an event were observed till their last date of follow-up. Patients who were lost to follow-up had their last known date of follow-up recorded. The study was approved by Human Research Ethics Committee at University of Witwatersrand, Johannesburg (study clearance number is M22111177 MED21-10-068).
Study population
All patients above 18 years of age who attended the LT clinic at the Wits Donald Gordon Medical Centre between 1st August 2004 to 31st August 2019 were included. Exclusion criteria included age < 18 years, LT for causes other than AIH, AIH overlap syndromes and transplantation at other centres. The minimum follow-up period was 12-months post transplantation. AIH was initially diagnosed based on the revised scoring system of the IAIHG.
End points
The primary end-point was to determine the 1- and 5- year patient and graft survival. The secondary aim was to determine the association between race and rejection/survival rates.Statistical analysis
The results were analysed using Statistical Package for Social Sciences version 28. All data was tabulated in Microsoft Excel spread sheet and Redcap. Continuous data are reported as mean or median values. Categorical data are reported as proportions/percentages. Kaplan-Meier analysis (log-rank test) was undertaken to evaluate patient and graft survival. Death or graft loss were defined as the study end-points.
Of the 56 LTs performed for AIH, 38 patients had AIH on explant histology. Seventeen had AIH-overlap syndrome and one patient had alcoholic steatohepatitis and were excluded from the study.
Patient characteristics
Of the 38 patients, 78% (n = 30/38) were female with a mean age at transplantation of 37 years. Forty-two percent (n = 16/38) were black-African, 42% (n = 16/38) were Caucasian and 16% (n = 6/38) were of mixed multiracial ethnic communities. The average length in days from listing to transplant was 156 days. Slightly longer periods were noticed for black-Africans (185 days) as compared to Caucasians (120 days). The average Model for End-Stage Liver Disease (MELD) score on presentation was 18.5.
Donor characteristics
All patients received a deceased donor transplant. Of the deceased donors, equal number of male and female donors were found. Twenty-eight donors were of Caucasian ethnicity, seven were black-Africans and three of mixed multiracial ethnicity. All organ donors were cadaver, there were no organ donations after circulatory death during this period.
Transplant risk factors
Variables other than ethnicity were looked at to determine whether they influence the outcome of LT.
Recipient risk factors such as age at the time of transplant, sex, ethnicity, comorbidities including HIV antibody testing, ABO compatibility, Antinuclear Antibodies (ANA), Anti-Smooth Muscle Antibodies (ASMA), anti-Liver Kidney Microsome type-1 antibodies (anti-LKM-1) and Immunoglobulin G (IgG) concentrations (before transplant) were looked at.
Among the donor risk factors, data such as age at the time of transplant, ethnicity, ABO compatibility, donor type (living-related, living non-related or cadaver) and antibodies to Cytomegalovirus (CMV) and HIV was collected. Haplotyping and analysis of donor Killer Immunoglobulin-like Receptor (KIR) is not a routine part of our transplant work-up for donors or recipients.
Transplant risk factors such as waitlist time and cold ischemic times were analyzed. It is difficult for us to comment on the wait-list mortality rate for black-Africans for the time period of our study as data keeping for wait-list only dates back to 2017. The average cold ischemic time for all our transplants was 4 hours - 5 hours and did not exceed beyond 12 hours for any of our transplants.
Given our small sample size and missing data a multivariate analysis to determine whether other factors have a significant impact on the outcome of LT was not possible.
Rejection
Acute rejection episodes were qualified and quantified using the Banff scoring system. Forty-four percent (17/38) of the patients showed biopsy proven acute or chronic rejection. Of the 17 patients with acute rejection, five progressed to chronic rejection. The average Banff score was higher for black-Africans (6.7) as compared to Caucasians (4.6). IgG was found to be high in eight patients with acute and two patients with chronic rejection. Seventy percent of all rejection episodes (12/17) were seen in black-Africans. Of these 12 black patients, three patients experienced two episodes of rejection. Four of the 12 black-Africans with acute rejection were resistant to therapy with steroids. One female with steroid-resistant rejection required a retransplant which was also unsuccessful. No Caucasian patients in our cohort were found to be steroid-resistant. The rate of rejection among black-Africans was four times higher than in Caucasians.
Recurrence
Ten percent (n = 4/38) of all transplant patients showed evidence of recurrent AIH on histology. These were all black-African females and under 30 years of age.
Patient and graft survival
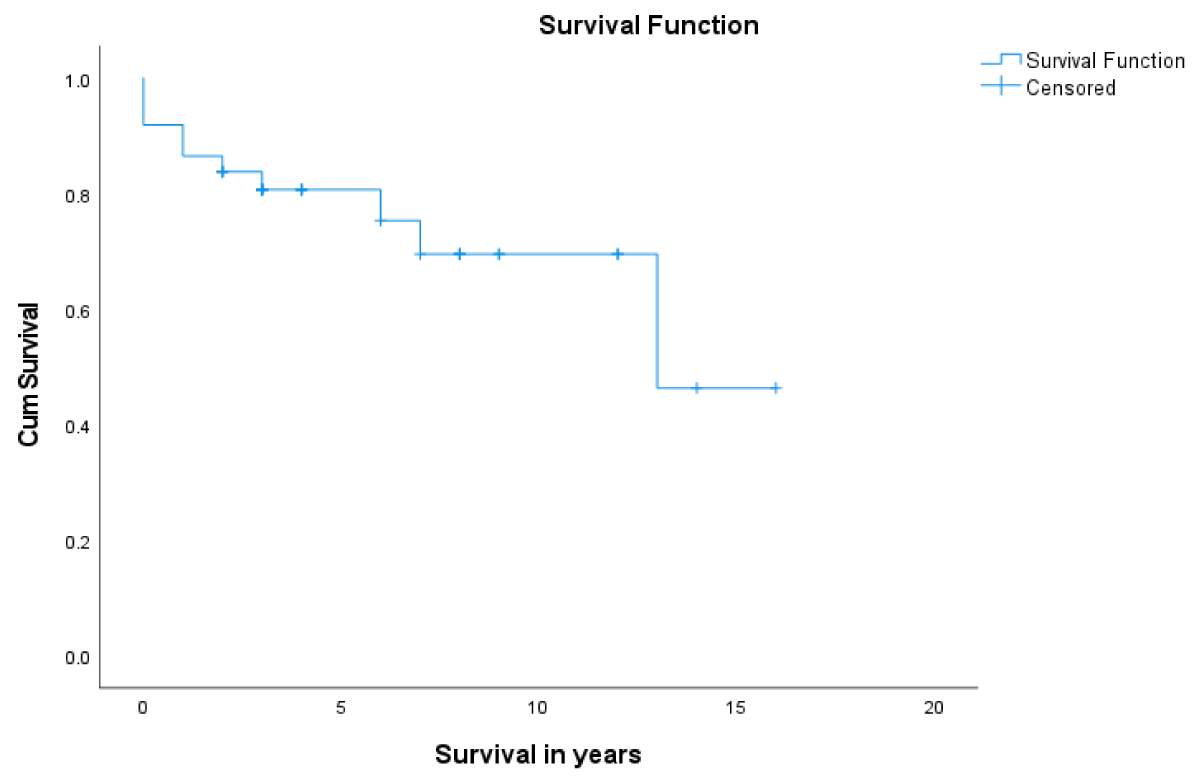
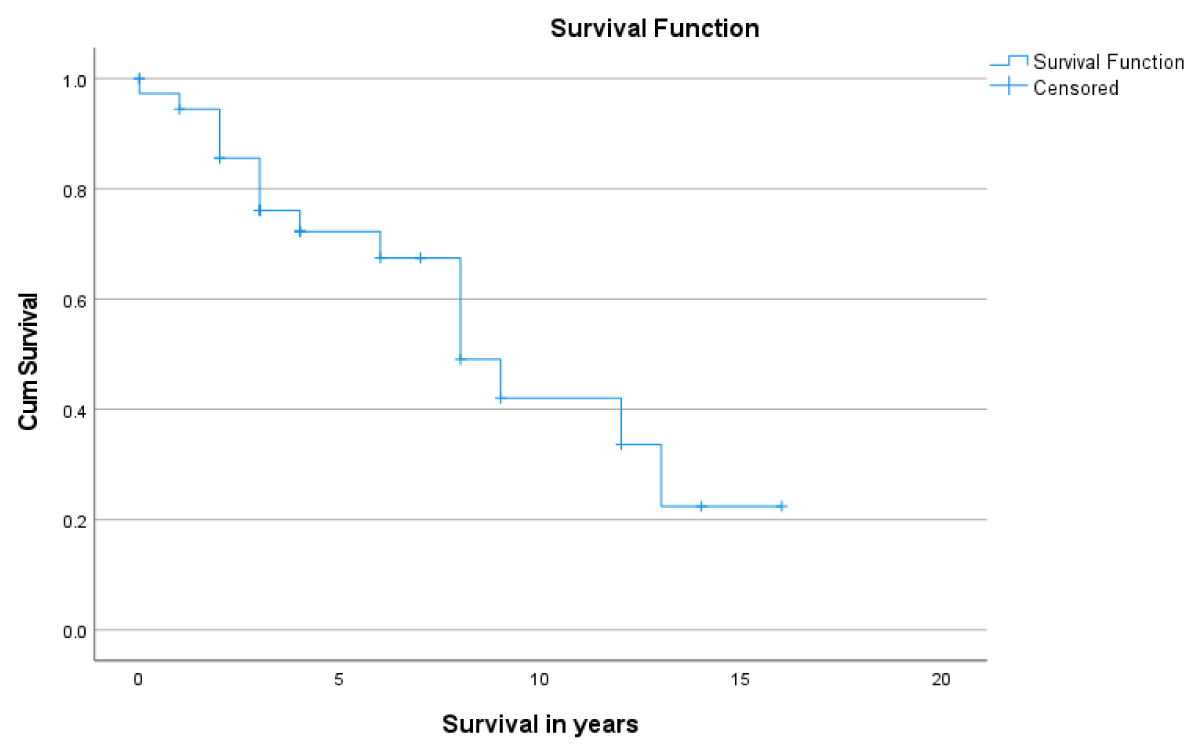
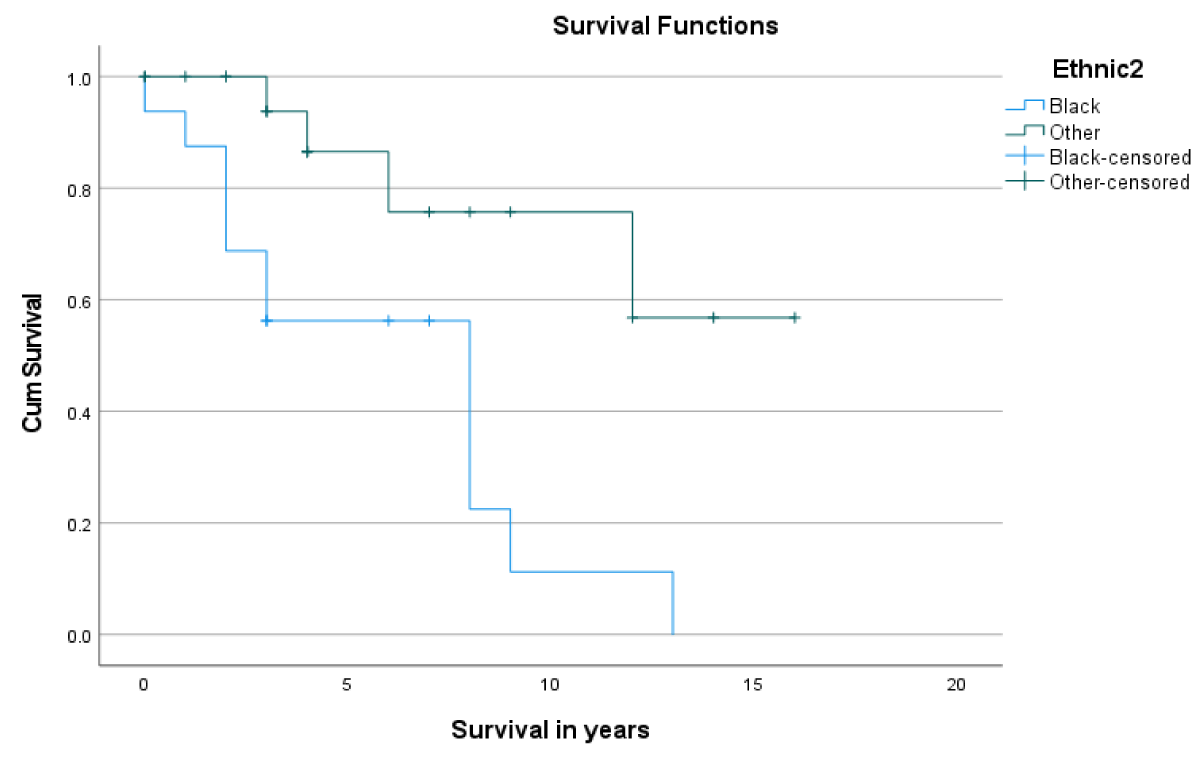
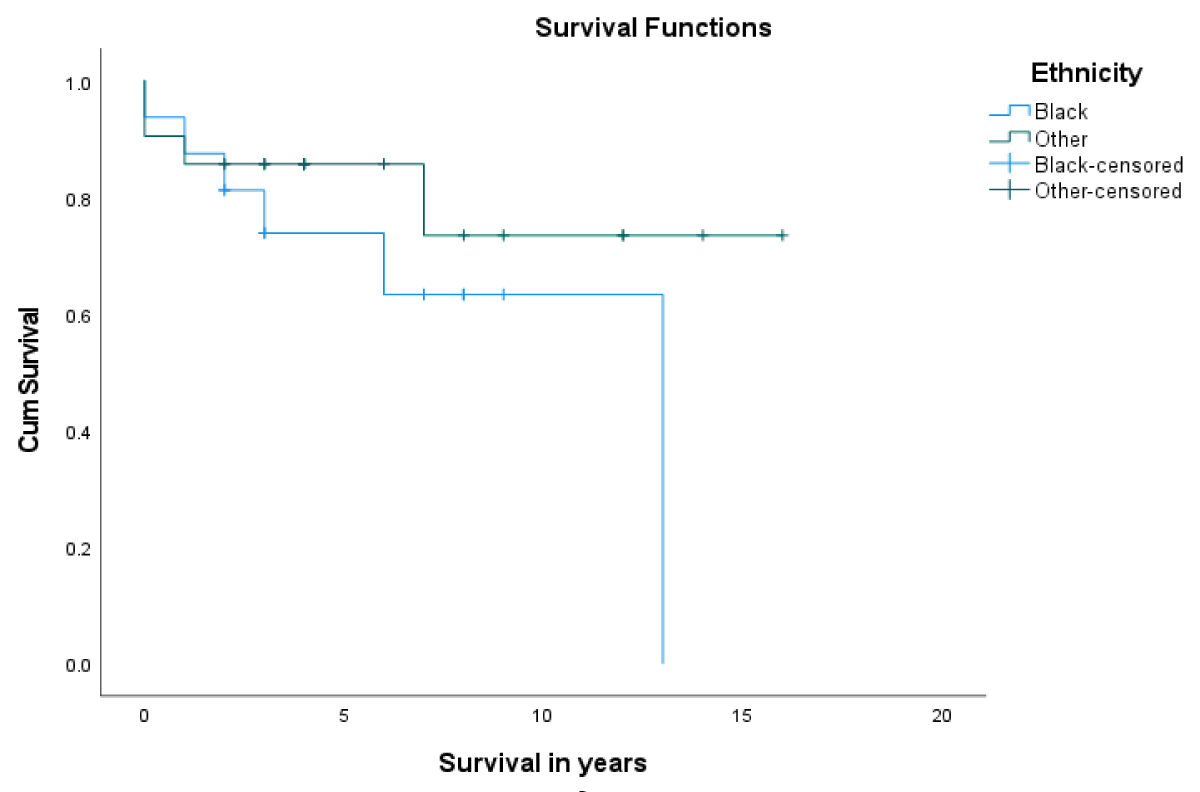
Post LT-patient survival at 1- and 5- years was 86.5% and 80.7%, respectively (Figure 1). Overall, 1- & 5- year graft survival was 94% and 70.8% respectively (Figure 2). The 1-year graft survival rate was 87.5% for black-Africans and 100% for Caucasians. The 5-year graft survival rate was 55% for black-Africans and 84.8% for Caucasians. (p - value 0.003 CI 3.8-8.1) (Figure 3). There was an insignificant 1- & 5- year patient survival difference between black-Africans (73.9%) and Caucasians (83.7%) (p - value 0.26, CI 6.3-12.2) (Figure 4).
Figure 1: 1- and 5-year patient survival post-LT.
Figure 2: 1- and 5-year graft survival post-LT.
Figure 3: 1- and 5- year graft survival of black-Africans as compared to Caucasians.
Figure 4: 1- and 5-year patient survival of black-Africans compared to Caucasians.
In this single-centre retrospective study, over a 15-year study period we identified 434 LT patients using the Redcap database. Twelve percent (n = 56/434) of all LT were indicated for AIH and 8% (n = 38/434) had AIH on explant histology. This rate was slightly higher than other cohorts in Europe and America (3%-6%) [17]. Our 1- & 5- year patient survival rates of 86.5% and 80.7%, respectively, are comparable to cohorts reported from European and American studies [13,14]. Our 1- & 5-year graft survival was significantly lower for black-Africans as compared to Caucasians and merits further investigation.
The success of LT is multifactorial. Black race is an independent risk factor for poor outcomes post-LT [18]. Studies have shown that black patients with AIH are at an increased risk for hospitalizations and post-LT mortality [18-21]. The lower 5-year patient and graft survival in our study is consistent with findings reported in other studies. A landmark study published in 2002 showed that black patients, regardless of their underlying liver disease, had a lower 2- and 5- survival after LT as compared to Caucasians [17]. Similarly in a study done by Thuluvath, et al. [16], African Americans with AIH had a lower 5-year survival compared to Caucasians.
The lower long-term survival among black-Africans could be attributed to socioeconomic factors. In South Africa social issues are prevalent and may contribute to different outcomes. However, in order to attribute the lower survival on socio-economic factors, these variables need to be further explored on an individual basis.
Black-Africans were found to have longer waiting periods for LT. There was no significant difference in the post-transplant 1- & 5- year patient survival, however the 1- & 5- year graft survival was found to be significantly lower. Interestingly, other studies also report on healthcare disparities where black patients were reported to have either low listing rates or demised while on the wait-list for transplant [22,23]. The severity of disease on presentation was unlikely to be a contributing factor as the average MELD scores among the two groups were similar (19 for black-Africans and 18.5 for Caucasians).
The longer wait-list periods noted for black-Africans were unlikely due to ABO incompatibility. In our cohort of 38 patients, majority of black-African recipients were O positive. It was also the most common blood group in our donor cohort.
Twelve of the 17 patients who experienced rejection were black-Africans. A rate significantly higher as compared to Caucasians. Four of the twelve black-Africans who had acute rejection were resistant to treatment with high dose steroids. One of these patients required a retransplant soon after her first transplant which was also unsuccessful. We aim to treat rejection by pulsing with high dose (1 gram) intravenous methylprednisone for three days as an inpatient. If concern remains, we perform a biopsy usually on the fourth day. In case of an active rejection, we repeat another three-day cycle of high dose methylprednisone and a repeat biopsy if still concerned for ongoing rejection. Anti-thymoglobulin is then given for ongoing rejection. Two of the steroid resistant patients proceeded to chronic rejection. Maggard, et al. [24] also reported a higher incidence of rejection among black LT recipients, however no cause was established in that study. In another study Vasquez, et al. reported higher failure rates with corticosteroid treatment for acute rejection among African-American which led to significantly lower 1- year survival among African-Americans (78%) as compared to non-African-Americans (96%) [25]. The higher rate of rejection and resistance to corticosteroids could perhaps be due to different pharmacodynamics in black patients. Genetic polymorphisms of the cytochrome complex in the black population are well defined. Cytochrome P450 3A5 is mainly responsible for the metabolism of Calcineurin Inhibitors (CNIs). Its polymorphism is present in 55% of the black population [26]. In-vitro studies among transplant recipients showed that black patients have reduced immunosuppressive effects on tacrolimus-based regimens due to enhanced immunoreactivity [27]. This is probably due to lower trough levels of CNIs among this population which lead to allograft rejection [28-30].
Our practice aims for an average tacrolimus trough level of 10 nanogram per millilitre (ng/mL) within the first three months post-transplant. In our cohort of 38 patients, tacrolimus levels were only found for 30 patients due to missing data. Of these 30 patients, 15 patients-maintained levels of 10 ng/ml and the other half had average levels of 7 ng/ml. Given our small sample size and missing data, we are unable to comment on whether lower tacrolimus levels influenced graft survival. However, we did notice higher doses of tacrolimus were required to maintain a trough level of 10ng/mL in two black-African patients. Even after the desired trough levels were achieved both these patients proceeded to develop chronic rejection. Due to limited resources, it is not common practice to test our patients for polymorphisms of drug metabolizing enzymes, however routine availability of such tests could significantly impact the treatment strategies and outcome of our transplant recipients.
Four of the 38 patients had histopathological features compatible with recurrent AIH. AIH recurrence post-transplant is well-defined, with a prevalence of 17% - 42% [31]. It is a major cause of graft dysfunction and reduced graft survival requiring re-transplantation. Apart from established risk factors such as younger age, higher IgG levels pre-transplant, donor sex mismatch and use of MMF, recipients with HLA-DRB1*0301 or DRB1*0401 are also known to be at higher risk for recurrence [32]. All of the patients in our cohort with recurrence were black-African females less than 30 years of age. Three of the four received mycofenotilmofetil (MMF) post-transplant and had Caucasian male donors. A high level of pre-transplant IgG was found in one of the patients and the other three did not have documented levels. Our standard practice to treat recurrence is high dose steroids, an increased dose of tacrolimus and change of regimen from MMF to Azathiopurine (AZA).
Our immunosuppressive regimens were same across all ethnicities. Our induction therapy includes high dose of intravenous steroids (methylprednisone) gradually weaned to oral prednisone over a period of 5 days. Tacrolimus is also given as part of induction at a dose of 0.1mg/kg. Humanized monoclonal antibody (interleukin-2 receptor inhibitor) basiliximab is a recent addition to our induction protocol since 2019. Only one of the four patients who were transplanted after 2019 received the drug. This is likely due to misdiagnosis pre-transplant and AIH only being confirmed on explant histology. Our standard maintenance therapy is triple regimen for all patients with AIH. We prefer to continue life-long steroids which are usually reduced to a dose of 7.5 mg - 10 mg/day after a period of three months. In a 5-year follow-up of patients in our cohort, MMF, tacrolimus and prednisone was to be the most common regimen. We don’t routinely use mammalian target of rapamycin (mTOR) inhibitors and only two patients in our cohort required it’s use. Of late, our clinical practice has changed with the increasing use of AZA instead of MMF. This is due to a recent study which showed an increased risk of recurrence with the use of MMF [31]. However, given the established difference of pharmacodynamics among the black population, a concern remains if a significant finding in a majority Caucasian population would have the same effect on a pre-dominantly black population.
The gene-environmental interplay is well defined in the pathogenesis of AIH [33]. Data on genetics is mostly available for Caucasian, Chinese and Japanese populations. There is paucity of data from the sub-Saharan population. Genetic predisposition to AIH has been related to several genes within and outside of HLA system. HLA genes reside on Major Histocompatibility Complex (MHC) on the short arm of chromosome 6 [34]. DRB1 allelic variants found within the HLA genes are responsible for the susceptibility and resistance to AIH [8]. American, Asian and European studies have described geographic variations within HLA alleles associated with AIH. Further studies have shown that different variants of DRB alleles are associated with resistance in different populations [35-37]. Outside the HLA system, tumour necrosis factor-induced protein 3 and cytotoxic T lymphocyte associated protein 4 (CTLA-4) have been associated with the development of AIH predominantly in the Chinese population [38]. A South African study done in 2008 did not find a novelty mutation in CTLA-4 gene among black-Africans with AIH [39]. To our knowledge no other studies have since been conducted in South Africa.
We report on the 1- & 5- year post-LT survival rate of South African population with AIH. Our patient survival rates are similar to data reported from European and American registries. However, our observation continues to suggest a more challenging post-transplant course among black-Africans. This is evident by a significantly lower 5-year graft survival, higher rate of rejection, recurrence and steroid resistance with potential development of chronic rejection noted among this population. Even though the limitation of our study is a small sample size, these findings are significant enough to warrant further investigation.
AIH differs in occurrence, phenotype and outcome for different populations. It suggests that other triggers might exist among the black population that have not yet been described.
The underlying genetics of black-Africans could be responsible for the severity and lower survival and is in fact a growing area of research. The results of genetic studies provide an explanation for the phenotypic heterogeneity of AIH among other populations but data from the African population is scarce.
In future, we aim to conduct larger studies to explore the genetics of black-Africans with AIH. Such an understanding could potentially contribute to individualized therapy and enhance outcomes of patients with AIH in South Africa.
Limitations
The limitation of this retrospective study is a small sample size. A retrospective records review depends entirely on the quality and accuracy of record keeping. In this case not all data planned to be collected were available in patients files.
Dr. June Fabian, Heather Maher, Research unit at Donald Gordon Medical Center, Department of Gastroenterology & Hepatology, Charlotte Maxeke Johannesburg Academic Hospital.
- Liberal R, Zen Y, Mieli-Vergani G, Vergani D. Liver transplantation and autoimmune liver diseases. Liver Transpl. 2013 Oct;19(10):1065-77. doi: 10.1002/lt.23704. Epub 2013 Aug 13. PMID: 23873751.
- Bernal RB, Medina-MoralesE, Goyes D. Management of autoimmune liver diseases after liver transplantation, Transplantology. 2021; 2:162-182.
- Taubert R, Hupa-Breier KL, Jaeckel E, Manns MP. Novel therapeutic targets in autoimmune hepatitis. J Autoimmun. 2018 Dec;95:34-46. doi: 10.1016/j.jaut.2018.10.022. Epub 2018 Nov 4. PMID: 30401504.
- Carbone M, Neuberger JM. Autoimmune liver disease, autoimmunity and liver transplantation. J Hepatol. 2014 Jan;60(1):210-23. doi: 10.1016/j.jhep.2013.09.020. Epub 2013 Sep 29. PMID: 24084655.
- Padilla M, Mayorga R, Carrasco F, Bedregal T, Bobadilla F, Rondón C, Chaman J. Liver transplantation for autoimmune hepatitis in Peru: outcomes and recurrence. Ann Hepatol. 2012 Mar-Apr;11(2):222-7. PMID: 22345339.
- Song E, Fabian J, Boshoff PE, Maher H, Gaylard P, Bentley A, Hale MJ, Ngwenya SP, Etheredge H, Mahomed A, Bobat B, Strobele B, Loveland J, Britz R, Botha JF. Adult liver transplantation in Johannesburg, South Africa (2004 - 2016): Balancing good outcomes, constrained resources and limited donors. S Afr Med J. 2018 Oct 26;108(11):929-936. doi: 10.7196/SAMJ.2018.v108i11.13286. PMID: 30645959.
- Krawitt EL. Autoimmune hepatitis. N Engl J Med. 2006 Jan 5;354(1):54-66. doi: 10.1056/NEJMra050408. PMID: 16394302.
- Sucher E, Sucher R, Gradistanac T, Brandacher G, Schneeberger S, Berg T. Autoimmune Hepatitis-Immunologically Triggered Liver Pathogenesis-Diagnostic and Therapeutic Strategies. J Immunol Res. 2019 Nov 25;2019:9437043. doi: 10.1155/2019/9437043. PMID: 31886312; PMCID: PMC6899271.
- Schneider JS, Montani M, Stickel F. Drug-Induced Autoimmune Hepatitis following Treatment with Zoledronic Acid. Case Rep Gastroenterol. 2017 Aug 8;11(2):440-445. doi: 10.1159/000479314. PMID: 29033761; PMCID: PMC5624238.
- Mubder M, Azab M, Jayaraj M, Cross C, Lankarani D, Dhindsa B, Pan JJ, Ohning G. Autoimmune hepatitis in patients with human immunodeficiency virus infection: A systematic review of the published literature. Medicine (Baltimore). 2019 Sep;98(37):e17094. doi: 10.1097/MD.0000000000017094. PMID: 31517833; PMCID: PMC6750342.
- Robinson MA, Nagurla SR, Noblitt TR, Almaghlouth NK, Al-Rahamneh MM, Cashin LM. Falsely positive fourth generation ADVIA Centaur® HIV Antigen/Antibody Combo assay in the presence of autoimmune hepatitis type I (AIH). IDCases. 2020 Jun 25;21:e00886. doi: 10.1016/j.idcr.2020.e00886. PMID: 32642434; PMCID: PMC7334457.
- Gleeson D. Long-Term Outcomes of Autoimmune Hepatitis. Clin Liver Dis (Hoboken). 2019 Aug 2;14(1):24-28. doi: 10.1002/cld.797. PMID: 31391933; PMCID: PMC6677096.
- Heinemann M, Adam R, Berenguer M, Mirza D, Malek-Hosseini SA, O'Grady JG, Lodge P, Pratschke J, Boudjema K, Paul A, Zieniewicz K, Fronek J, Weiss KH, Karam V, Duvoux C, Lohse A, Schramm C; all the other contributing centers (www.eltr.org) and the European Liver and Intestine Transplant Association (ELITA). Longterm Survival After Liver Transplantation for Autoimmune Hepatitis: Results From the European Liver Transplant Registry. Liver Transpl. 2020 Jul;26(7):866-877. doi: 10.1002/lt.25739. Epub 2020 May 1. PMID: 32112516.
- Vogel A, Heinrich E, Bahr MJ, Rifai K, Flemming P, Melter M, Klempnauer J, Nashan B, Manns MP, Strassburg CP. Long-term outcome of liver transplantation for autoimmune hepatitis. Clin Transplant. 2004 Feb;18(1):62-9. doi: 10.1111/j.1399-0012.2004.00117.x. PMID: 15108772.
- Lim KN, Casanova RL, Boyer TD, Bruno CJ. Autoimmune hepatitis in African Americans: presenting features and response to therapy. Am J Gastroenterol. 2001 Dec;96(12):3390-4. doi: 10.1111/j.1572-0241.2001.05272.x. PMID: 11774954.
- Thuluvath PJ, Wagennar RR, Verma S. Gender and ethnic differences in the post-liver transplant outcomes of patients with autoimmune hepatitis with acute liver failure at initial presentation: a case-control study. Arch Med Sci. 2015 Dec 10;11(6):1227-35. doi: 10.5114/aoms.2015.52736. Epub 2015 Dec 11. PMID: 26788084; PMCID: PMC4697044.
- Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM; American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010 Jun;51(6):2193-213. doi: 10.1002/hep.23584. PMID: 20513004.
- Nair S, Eustace J, Thuluvath PJ. Effect of race on outcome of orthotopic liver transplantation: a cohort study. Lancet. 2002 Jan 26;359(9303):287-93. doi: 10.1016/S0140-6736(02)07494-9. PMID: 11830194.
- Lee BP, Dodge JL, Terrault NA. Changes and mediators of survival disparity among Black liver transplant recipients in the United States. Am J Transplant. 2021 Dec;21(12):3883-3893. doi: 10.1111/ajt.16767. Epub 2021 Aug 10. PMID: 34374495.
- Bayable A, Liu B, Bhuket T. 1001 significant differences in liver transplant (LT) waitlist mortality, receiving LT, and post-LT survival among adults with primary sclerosing cholangitis, primary biliary cholangitis, and autoimmune hepatitis in the U.S. Am J Gastroenterol. 2019; 114: S581.
- Wen JW, Kohn MA, Wong R, Somsouk M, Khalili M, Maher J, Tana MM. Hospitalizations for Autoimmune Hepatitis Disproportionately Affect Black and Latino Americans. Am J Gastroenterol. 2018 Feb;113(2):243-253. doi: 10.1038/ajg.2017.456. Epub 2018 Jan 30. PMID: 29380822; PMCID: PMC6522224.
- Rosenblatt R, Wahid N, Halazun KJ, Kaplan A, Jesudian A, Lucero C, Lee J, Dove L, Fox A, Verna E, Samstein B, Fortune BE, Brown RS Jr. Black Patients Have Unequal Access to Listing for Liver Transplantation in the United States. Hepatology. 2021 Sep;74(3):1523-1532. doi: 10.1002/hep.31837. PMID: 33779992.
- Nsubuga JP, Goyes D, Trivedi HD, Medina-Morales E, Patwardhan V, Bonder A. Waitlist Mortality and Posttransplant Outcomes in African Americans with Autoimmune Liver Diseases. J Transplant. 2021 Aug 3;2021:6692049. doi: 10.1155/2021/6692049. PMID: 34394979; PMCID: PMC8357471.
- Maggard M, Goss J, Ramdev S, Swenson K, Busuttil RW. Incidence of acute rejection in African-American liver transplant recipients. Transplant Proc. 1998 Jun;30(4):1492-4. doi: 10.1016/s0041-1345(98)00330-3. PMID: 9636607.
- Vasquez EM, Benedetti E, Pollak R. Ethnic differences in clinical response to corticosteroid treatment of acute renal allograft rejection. Transplantation. 2001 Jan 27;71(2):229-33. doi: 10.1097/00007890-200101270-00010. PMID: 11213064.
- Lamba JK, Lin YS, Schuetz EG, Thummel KE. Genetic contribution to variable human CYP3A-mediated metabolism. Adv Drug Deliv Rev. 2002 Nov 18;54(10):1271-94. doi: 10.1016/s0169-409x(02)00066-2. PMID: 12406645.
- Nagashima N, Watanabe T, Nakamura M, Shalabi A, Burdick JF. Decreased effect of immunosuppression on immunocompetence in African--Americans after kidney and liver transplantation. Clin Transplant. 2001 Apr;15(2):111-5. doi: 10.1034/j.1399-0012.2001.150207.x. PMID: 11264637.
- Min DI, Lee M, Ku YM, Flanigan M. Gender-dependent racial difference in disposition of cyclosporine among healthy African American and white volunteers. Clin Pharmacol Ther. 2000 Nov;68(5):478-86. doi: 10.1067/mcp.2000.111255. PMID: 11103750.
- Isaacs RB, Nock SL, Spencer CE, Connors AF Jr, Wang XQ, Sawyer R, Lobo PI. Racial disparities in renal transplant outcomes. Am J Kidney Dis. 1999 Oct;34(4):706-12. doi: 10.1016/S0272-6386(99)70397-5. PMID: 10516353.
- Zahir H, McCaughan G, Gleeson M, Nand RA, McLachlan AJ. Factors affecting variability in distribution of tacrolimus in liver transplant recipients. Br J Clin Pharmacol. 2004 Mar;57(3):298-309. doi: 10.1046/j.1365-2125.2003.02008.x. PMID: 14998426; PMCID: PMC1884454.
- Montano-Loza AJ, Ronca V, Ebadi M, Hansen BE, Hirschfield G, Elwir S, Alsaed M, Milkiewicz P, Janik MK, Marschall HU, Burza MA, Efe C, Calışkan AR, Harputluoglu M, Kabaçam G, Terrabuio D, de Quadros Onofrio F, Selzner N, Bonder A, Parés A, Llovet L, Akyıldız M, Arikan C, Manns MP, Taubert R, Weber AL, Schiano TD, Haydel B, Czubkowski P, Socha P, Ołdak N, Akamatsu N, Tanaka A, Levy C, Martin EF, Goel A, Sedki M, Jankowska I, Ikegami T, Rodriguez M, Sterneck M, Weiler-Normann C, Schramm C, Donato MF, Lohse A, Andrade RJ, Patwardhan VR, van Hoek B, Biewenga M, Kremer AE, Ueda Y, Deneau M, Pedersen M, Mayo MJ, Floreani A, Burra P, Secchi MF, Beretta-Piccoli BT, Sciveres M, Maggiore G, Jafri SM, Debray D, Girard M, Lacaille F, Lytvyak E, Mason AL, Heneghan M, Oo YH; International Autoimmune Hepatitis Group (IAIHG). Risk factors and outcomes associated with recurrent autoimmune hepatitis following liver transplantation. J Hepatol. 2022 Jul;77(1):84-97. doi: 10.1016/j.jhep.2022.01.022. Epub 2022 Feb 8. PMID: 35143897.
- Edmunds C, Ekong UD. Autoimmune Liver Disease Post-Liver Transplantation: A Summary and Proposed Areas for Future Research. Transplantation. 2016 Mar;100(3):515-24. doi: 10.1097/TP.0000000000000922. PMID: 26447505; PMCID: PMC4764021.
- Lammert C. Genetic and Environmental Risk Factors for Autoimmune Hepatitis. Clin Liver Dis (Hoboken). 2019 Aug 2;14(1):29-32. doi: 10.1002/cld.798. PMID: 31391934; PMCID: PMC6677013.
- Higuchi T, Oka S, Furukawa H, Tohma S, Yatsuhashi H, Migita K. Genetic risk factors for autoimmune hepatitis: implications for phenotypic heterogeneity and biomarkers for drug response. Hum Genomics. 2021 Jan 28;15(1):6. doi: 10.1186/s40246-020-00301-4. PMID: 33509297; PMCID: PMC7841991.
- Yoshizawa K, Ota M, Katsuyama Y, Ichijo T, Matsumoto A, Tanaka E, Kiyosawa K. Genetic analysis of the HLA region of Japanese patients with type 1 autoimmune hepatitis. J Hepatol. 2005 Apr;42(4):578-84. doi: 10.1016/j.jhep.2004.12.019. Epub 2005 Jan 21. PMID: 15763345.
- Bittencourt PL, Goldberg AC, Cançado EL, Porta G, Carrilho FJ, Farias AQ, Palacios SA, Chiarella JM, Abrantes-Lemos CP, Baggio VL, Laudanna AA, Kalil J. Genetic heterogeneity in susceptibility to autoimmune hepatitis types 1 and 2. Am J Gastroenterol. 1999 Jul;94(7):1906-13. doi: 10.1111/j.1572-0241.1999.01229.x. PMID: 10406258.
- Strettell MD, Donaldson PT, Thomson LJ, Santrach PJ, Moore SB, Czaja AJ, Williams R. Allelic basis for HLA-encoded susceptibility to type 1 autoimmune hepatitis. Gastroenterology. 1997 Jun;112(6):2028-35. doi: 10.1053/gast.1997.v112.pm9178696. PMID: 9178696.
- Eskandari-Nasab E, Tahmasebi A, Hashemi M. Meta-analysis: the relationship between CTLA-4 +49 A/G polymorphism and primary biliary cirrhosis and type I autoimmune hepatitis. Immunol Invest. 2015;44(4):331-48. doi: 10.3109/08820139.2014.1003651. PMID: 25942345.
- Marais S. CTLA4 Gene Polymorphisms in Autoimmune Hepatitis (AIH): Gene and Clinical Disease Correlation in South African Patients. MSc (Med). University of Cape Town, Cape Town. 2007.