More Information
Submitted: April 06, 2020 | Approved: May 17, 2022 | Published: May 18, 2022
How to cite this article: Yetisir F, Babayeva A, Güzel K. Laparoscopic surgical treatment of median arcuate ligament syndrome with the retrograde division technique: a case report. Ann Clin Gastroenterol Hepatol. 2022; 6: 021-024.
DOI: 10.29328/journal.acgh.1001034
Copyright License: © 2022 Yetisir F, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Median arcuate ligament syndrome; Celiac artery syndrome; Laparoscopic division of arcuate
Laparoscopic surgical treatment of median arcuate ligament syndrome with the retrograde division technique: a case report
Fahri Yetisir1*, Afruz Babayeva2 and Kerim Güzel3
1General Surgery Department, VM Medicalpark Ankara Private Hospital, Yüksek İhtisas University, Ankara, Turkey
2Endocrinology, Gazi University, Ankara, Turkey
3General Surgery Department, Near East University, Turkey
*Address for Correspondence: Dr. FahriYetişir, General Surgery Department, VM Medicalpark Ankara Private Hospital, Yüksek İhtisas University, Post code:06800, Ankara, Turkey, Email:[email protected]
Median arcuate ligament syndrome is a rare entity. This clinical condition develops by compression of the root of a celiac artery with the median arcuate ligament. The typical triad of this syndrome is the following; abdominal discomfort and pain, especially after a meal, and weight loss. In diagnosis, other causes should be ruled out and compression must be demonstrated by any type of imaging method. The main principle of treatment is cutting down the median arcuate ligament. A 54-year-old woman presented with untreatable recurrent abdominal pain and was diagnosed with median arcuate ligament syndrome by imaging with angiographic computed tomography. This patient was operated on. We performed laparoscopic division of median arcuate ligament with the retrograde surgical dissection technique. The patient was discharged from the hospital without any complaint on the third day after surgery. She was still symptom-free after 12 months.
The laparoscopic retrograde dissection approach is a safe and feasible treatment modality for median arcuate ligament syndrome.
Median arcuate ligament syndrome (MALS), also known as the celiac artery compression syndrome, Dunbar syndrome, or celiac axis syndrome, is a very rare clinical situation that is characterized by recurrent abdominal pain, especially after a meal and is often misdiagnosed due to its relative scarcity [1]. The median arcuate ligament (MAL) is the fibroid connective tissue that is attached to the right and left diaphragmatic crura on both sides of the aortic foramen. This MAL may cause extrinsic compression on the root of the celiac trunk if it is located lower position, or if the celiac artery originates higher position. The root of the celiac artery generally originates from the aorta below the MAL and makes the celiac trunk then separates into its branches. The MAL is very close to the celiac trunk in 10% - 24% of the population. The degree of compression on the artery relative to the position of the diaphragm. The degree of compression is maximized during the deep expiration. This compression to this artery reduces blood supply to the stomach, liver, spleen, and intestine, therefore mesenteric ischemia, and related symptoms [2]. The typical signs and symptoms of MALS are abdominal pain and discomfort especially after a meal, weight loss over a long period, and an abdominal vascular murmur. The other manifestations include nausea, vomiting, diarrhea, and anorexia. The diagnosis of MALS requires imaging studies and ruled out other causes. The primary treatment of MALS is the total division of the compressing parts of MAL to release the extrinsic compression on the root of the celiac artery. In recent years, frequently laparoscopic surgery has been accepted as a gold standard in the management of MALS [3].
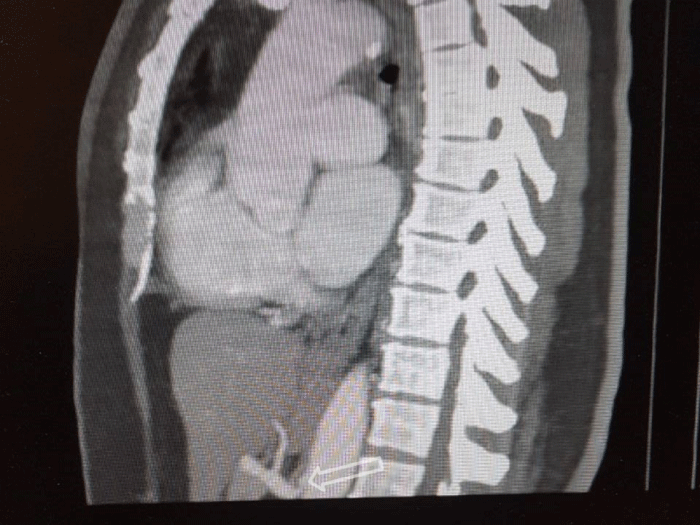
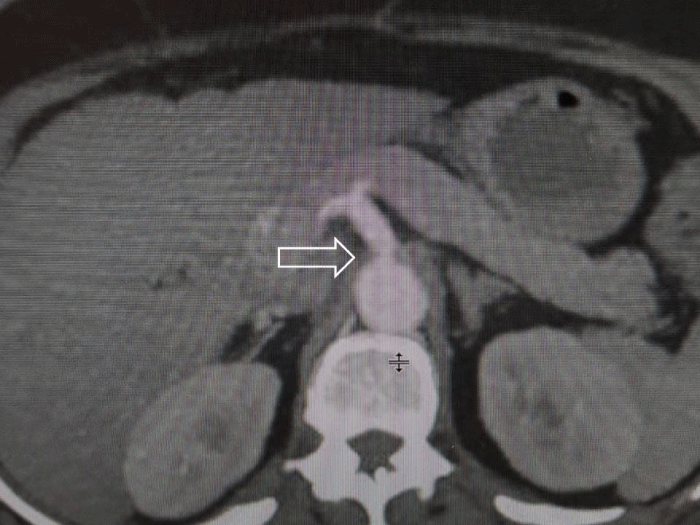
A 54-year-old patient presented with upper abdomen pain. She has had several times severe abdominal pain with different duration time, especially after a meal. She has had hypertension and depression for a few years. Blood investigations, abdomen ultrasound, and CT were normal. The results of esophagogastroduodenoscopy were normal. There is only epigastric tenderness in physical examination. An apparent compression of the root of the celiac axis by the MAL is shown in vertical and sagittal views of the CT angiography (Figures 1 and 2). Written informed consent was obtained from the patient who participated in this case and she underwent a laparoscopic operation for MALS.
Figure 1: Vertical section enhanced abdominal CT shows narrowing of celiac axis beyond its origin.
Figure 2: Sagittal section enhanced abdominal CT shows narrowing of celiac axis beyond its origin.
Surgical technique
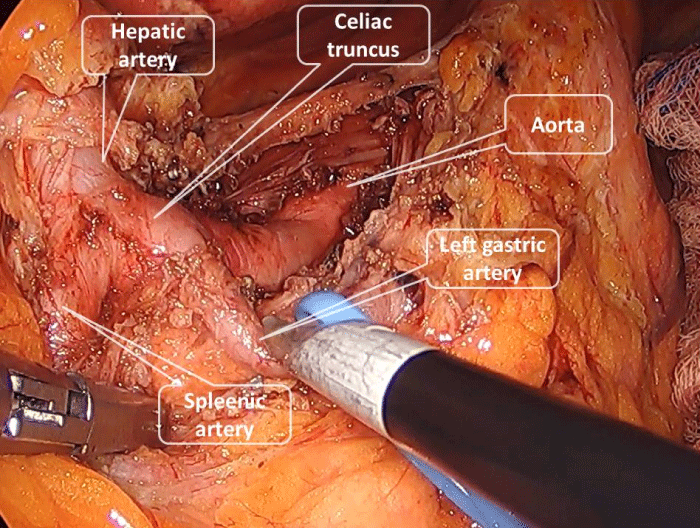
The patient was placed in a supine position with legs split. The first 10-mm camera port was placed at 3 cm above the umbilicus. A 5-mm epigastric port was placed through which an automatic liver retractor was placed to lift the left lobe of the liver. Two 5-mm ports were introduced in the right and left upper abdomen and an additional 10-mm port was introduced in the left lateral abdomen. We used a 30° scope. For good exposure, the stomach was retracted to the up and the left. After dividing pars flaccida crura was dissected. We used the laparoscopic retrograde division technique. Left gastric artery suspended with penrose drain to be able to see celiac truncus in a better manner. Our starting point was the left gastric artery. Dissection of MAL was performed below to upward by identifying the branches of the celiac truncus. After identifying the left gastric, splenic artery, and hepatic artery the celiac truncus and its root were dissected up to the aorta. All the fibrous tissue on either side of the celiac truncus, its rood, and origin was dissected gently and excised to provide sufficient dilation of the celiac origin. After the division of all fibrous tissue around the celiac artery, the dilated celiac axis was visualized without any narrowing on it (Figure 3) the operative time was 130 minutes and during the operation, no complications developed.
Figure 3: Entire celiac axis bared of all fibro-fatty tissue. All branches of celiac truncus are shown in this picture.
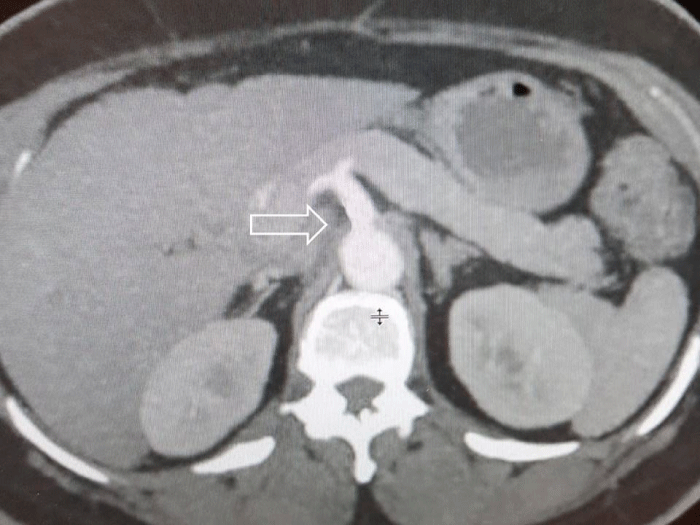
All the symptoms of MALS disappeared within 3 days and the patient was discharged from the hospital. At the 12 months after the operation enhanced abdominal CT shows that the narrowing part of the celiac axis had improved (Figure 4).
Figure 4: Sagittal section enhanced abdominal CT shows improvement of the narrowing of celiac axis.
MALS is a very rare pathology as a result of extrinsic compression of the celiac artery by the median arcuate ligament, and nonspecific clinical presentation, so diagnosis is by exclusion. Differential diagnosis should be made to rule out the other disease related to the gastrointestinal system such as gallbladder disease, peptic ulcer, pancreatitis, appendicitis, inflammatory bowel disease, and chronic mesenteric ischemia. Postprandial epigastric ischemia and abdominal pain, and weight loss are often observed. The patient is usually thin body habitus, ages 20 to 40 years [2]. Abdominal pain is often presented after a meal or after exercise, especially at a specific body posture. In our case, the patient had no significant weight loss, and contrary to expectations was not slender but older.
However, due to persistent abdominal pain, diagnoses Computed tomography (CT) angiography of the patient was performed after exclusion of other gastrointestinal the diagnosis was made this way.
Stenosis of the celiac artery is compensated by collateral flow from the gastroduodenal branch of the superior mesenteric artery. The MALS may cause pancreatic duodenal arterial aneurysm and gastroparesis. A long-term extensive decrease in blood flow may be the main reason for the mechanism during long-term chronic ischemia [4,5]. Therefore, it is important to make a diagnosis before the complications develop. Doppler ultrasound is used for screening of MALS. The advantages of Doppler ultrasound is non-invasive and easy to perform, but the status of vessels cannot be assessed during the inspiration and expiration phase. The gold standard for diagnosis of MALS is angiography with the classic findings. Angiographic findings are a pathognomonic focal narrowing of the celiac trunk, especially during expiration, with or without post-stenotic dilatation [6]. Also, CT angiography and magnetic resonance angiography are non-invasive imaging forms to verify the location of the celiac trunk and compression [7]. In our case, the diagnosis of MALS was verified by CT angiography and the patient underwent surgery.
In the literature about the MALS, especially in symptomatic patients extraction of the fibrous structures and median arcuate ligament causing compression has been recommended [6,8]. The aim of treatment in MALS patients is to normalize celiac artery blood flow. Open or laparoscopic techniques can be made by cutting the median arcuate ligament. In addition, Patch angioplasty, aorta-celiac bypass, aortic reanastomosis of the celiac artery, and percutaneous endovascular initiatives can be made when the conventional methods are unsuccessful [9]. Today, laparoscopic surgery is more preferred than open surgery. The laparoscopic method has advantages such as, may reduce surgical trauma and patient hospitalization, less postoperative pain, and early oral intake. Doppler ultrasound may be used for confirming the passage of the celiac trunk [10].
The diagnoses of MALS are not so easy. Patients with MALS have nonspecific gastrointestinal symptoms. Establishing a causal link between the anatomic condition and the presence of symptoms may be a difficulty. As a result, MALS should be remembered during the differential diagnosis in patients with nausea and abdominal pain as the clinical presentation is mild and rare. Depending on the patient the treatment method might vary, but long-term follow-up is recommended as postoperative long-term complaints may reoccur in the future.
According to some authors, the interruption of somatic or sympathetic fibers during the release procedure of MAL may be the main actor in the improvement of symptoms of MALS. The mechanism is, blood flow is increased in the trough celiac artery due to perivascular denervation of the celiac ganglion due to relief in vasospasm. Until the last two decades, open conventional surgery was the gold standard therapy in the management of the MALS [11]. With technological development and minimally invasive surgery started to be used more commonly. In the last decades so many reports advocate the laparoscopic approach to MALS [12]. In the surgical technique, the dissection of MAL from below upward is more beneficial, starting from dissecting the left gastric artery and suspending it and going to the celiac root and the aorta is a more acceptable approach than identifying the aorta and tracing the celiac origin from above downward. With this technique, from above to downward, there is more risk to make an injure to the aorta and celiac artery [13].
Laparoscopic dissection of MAL provides better visualization of the aortic and celiac regions. In this way, complete division of all the fibers of MAL could be achieved easily. This is the key to the successful resolution of symptoms. The other advantages of minimally invasive surgery are less pain after surgery, faster recovery, early discharge, and early return to work [13].
The laparoscopic approach is the gold standard treatment modality for MALS with less morbidity and less mortality. The below upward dissection technique of MAL starting from a left gastric artery is more beneficial.
- Cienfuegos JA, Rotellar F, Valentí V, Arredondo J, Pedano N, Bueno A, Vivas I. The celiac axis compression syndrome (CACS): critical review in the laparoscopic era. Rev Esp Enferm Dig. 2010 Mar;102(3):193-201. English, Spanish. doi: 10.4321/s1130-01082010000300006. PMID: 20373834.
- Horton KM, Talamini MA, Fishman EK. Median arcuate ligament syndrome: evaluation with CT angiography. Radiographics. 2005 Sep-Oct;25(5):1177-82. doi: 10.1148/rg.255055001. PMID: 16160104.
- Cienfuegos JA, Estevez MG, Ruiz-Canela M, Pardo F, Diez-Caballero A, Vivas I, Bilbao JI, Martí-Cruchaga P, Zozaya G, Valentí V, Hernández-Lizoáin JL, Rotellar F. Laparoscopic Treatment of Median Arcuate Ligament Syndrome: Analysis of Long-Term Outcomes and Predictive Factors. J Gastrointest Surg. 2018 Apr;22(4):713-721. doi: 10.1007/s11605-017-3635-3. Epub 2017 Nov 28. PMID: 29185149.
- Trinidad-Hernandez M, Keith P, Habib I, White JV. Reversible gastroparesis: functional documentation of celiac axis compression syndrome and postoperative improvement. Am Surg. 2006 Apr;72(4):339-44. PMID: 16676860.
- Jimenez JC, Rafidi F, Morris L. True celiac artery aneurysm secondary to median arcuate ligament syndrome. Vasc Endovascular Surg. 2011 Apr;45(3):288-9. doi: 10.1177/1538574411399155. PMID: 21478249.
- Duffy AJ, Panait L, Eisenberg D, Bell RL, Roberts KE, Sumpio B. Management of median arcuate ligament syndrome: a new paradigm. Ann Vasc Surg. 2009 Nov-Dec;23(6):778-84. doi: 10.1016/j.avsg.2008.11.005. Epub 2009 Jan 6. PMID: 19128929.
- Sun Z, Zhang D, Xu G, Zhang N. Laparoscopic treatment of median arcuate ligament syndrome. Intractable Rare Dis Res. 2019 May;8(2):108-112. doi: 10.5582/irdr.2019.01031. PMID: 31218160; PMCID: PMC6557235.
- Baccari P, Civilini E, Dordoni L, Melissano G, Nicoletti R, Chiesa R. Celiac artery compression syndrome managed by laparoscopy. J Vasc Surg. 2009 Jul;50(1):134-9. doi: 10.1016/j.jvs.2008.11.124. PMID: 19563961.
- Hongsakul K, Rookkapan S, Sungsiri J, Tubtawee T. A severe case of median arcuate ligament syndrome with successful angioplasty and stenting. Case Rep Vasc Med. 2012;2012:129870. doi: 10.1155/2012/129870. Epub 2012 Sep 19. PMID: 23050191; PMCID: PMC3459250.
- Roayaie S, Jossart G, Gitlitz D, Lamparello P, Hollier L, Gagner M. Laparoscopic release of celiac artery compression syndrome facilitated by laparoscopic ultrasound scanning to confirm restoration of flow. J Vasc Surg. 2000 Oct;32(4):814-7. doi: 10.1067/mva.2000.107574. PMID: 11013046.
- Reilly LM, Ammar AD, Stoney RJ, Ehrenfeld WK. Late results following operative repair for celiac artery compression syndrome. J Vasc Surg. 1985 Jan;2(1):79-91. PMID: 3965762.
- Carbonell AM, Kercher KW, Heniford BT, Matthews BD. Multimedia article. Laparoscopic management of median arcuate ligament syndrome. Surg Endosc. 2005 May;19(5):729. doi: 10.1007/s00464-004-6010-x. PMID: 15965588.
- Wani S, Wakde V, Patel R, Patankar R, Mathur SK. Laparoscopic release of median arcuate ligament. J Minim Access Surg. 2012 Jan;8(1):16-8. doi: 10.4103/0972-9941.91775. PMID: 22303084; PMCID: PMC3267330.