More Information
Submitted: March 21, 2022 | Approved: April 12, 2022 | Published: April 13, 2022
How to cite this article: Yehuda R, Ramona NR. Pitfalls in the hemostatic management of a liver transplantation. Ann Clin Gastroenterol Hepatol. 2022; 6: 001-005.
DOI: 10.29328/journal.acgh.1001032
Copyright License: © 2022 Yehuda R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Pitfalls in the hemostatic management of a liver transplantation
Raveh Yehuda1,2* and Nicolau-Raducu Ramona1,2
1Department of Anesthesia, University of Miami/Jackson Memorial Hospital, Miami, FL, USA
2Miami Transplant Institute, University of Miami/Jackson Memorial Hospital, Miami, FL, USA
*Address for Correspondence: Yehuda Raveh, Department of Anesthesiology, Jackson Memorial Hospital, 1611 NW 12Th Avenue, DTC 318 Miami, FL, 33136, USA, Email: [email protected]
Liver Transplantation is fraught with thrombo-hemorrhagic complications, due to the precarious hemostasis of the recipient, anhepatic conditions, and the release of hemostatic factors from the allograft. Disseminated intravascular coagulation and its “flat-line” variant are common causes of hemorrhage and thrombosis, and frequently force the clinician along with a delicate balance between hemorrhage and thrombosis. We present a case that highlights some of the more challenging diagnostic and management decisions in liver transplantation and presents a safe and carefully structured approach to hyperfibrinolysis in liver transplantation.
The precarious hemostatic imbalance of cirrhosis is severely perturbed by Liver transplantation (LT) due to surgical trauma, anhepatic condition, and the release of pro-and anti-hemostatic factors from the allograft. As such, the procedure is fraught with life-threatening hemorrhagic and thrombotic complications [1-3], and ensuing morbidity and mortality [3,4]. To mitigate these risks, the transplant anesthesiologist is frequently required to navigate the patient along with a delicate balance between hemorrhage and thrombosis. Viscoelastic monitoring (VEM) has a crucial role in guiding the hemostatic management during LT [5], but the interpretation of point-of-care VEM is frequently nuanced. The discrimination between primary hyperfibrinolysis and secondary hyperfibrinolysis (i.e. disseminated intravascular coagulation (DIC)) during LT is both critical and challenging, as antifibrinolytics are indicated for the former [5,6], but may result in catastrophic thrombosis in DIC [6]. In addition, the surgical stage of the procedure should be a major factor when treating hemostatic derangements [7]. As one of the largest transplant centers in the nation and a front-runner's liver and intestinal/multivisceral transplant program our surgeons and anesthesiologists gained extensive experience in the diagnosis and management of intraoperative hemostasis. Delineated here is a case of LT that was associated with severe hemostatic abnormalities. The case illustrates the diagnostic and therapeutic decision-making as they pertain to the stages of the procedure, and is presented in a didactic commentary alongside the clinical scenario.
A 52-year-old Hispanic male was admitted for LT secondary to Laennec's cirrhosis and hepatocellular carcinoma. The patient had moderate ascites, and a wait-list Model of End-stage Liver Disease (MELD) score of 30. Recent computerized tomography revealed a non-occlusive thrombosis of the main and proximal right portal veins. Long-standing warfarin therapy was discontinued a week prior to admission. Laboratory results on admission showed INR 2; creatinine 1.1 mg/dL; platelet count 123 x 10^9 /L, and fibrinogen 172 mg/dL.
Ascitic fluid is a potent activator of coagulation and its enhanced absorption during LT perturbs hemostasis and triggers DIC [8,9]. Sakai, et al. found ascites and hemodynamic instability predictive of pulmonary embolism during LT [3]. In cirrhosis, portal vein thrombosis is secondary to a combination of reduced flow (Virchow’s triad) and hypercoagulability. The association of portal vein thrombosis with intraoperative hemostatic imbalance, however, was not clearly shown [10]. Prothrombin time, like all clot-based assays, is sensitive to procoagulants’ levels alone [11]. An elevated INR, therefore, reflects the magnitude of synthetic (hemostatic) hepatic failure, but not the actual balance between pro-and anticoagulants.
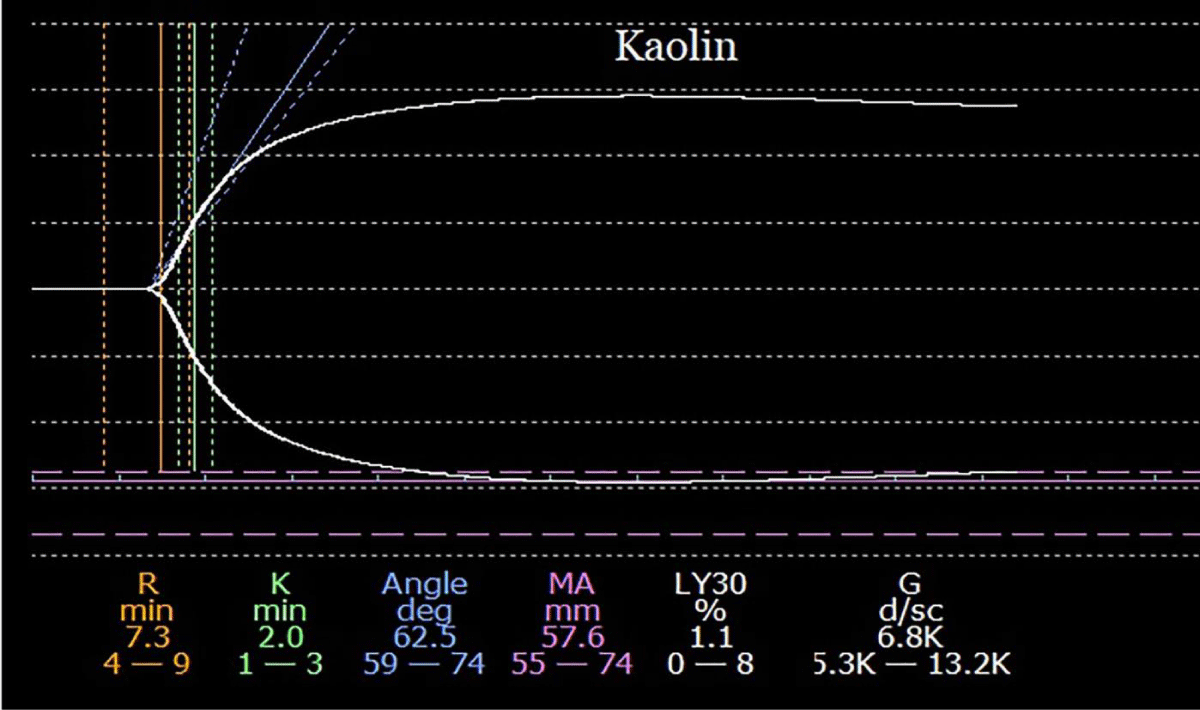
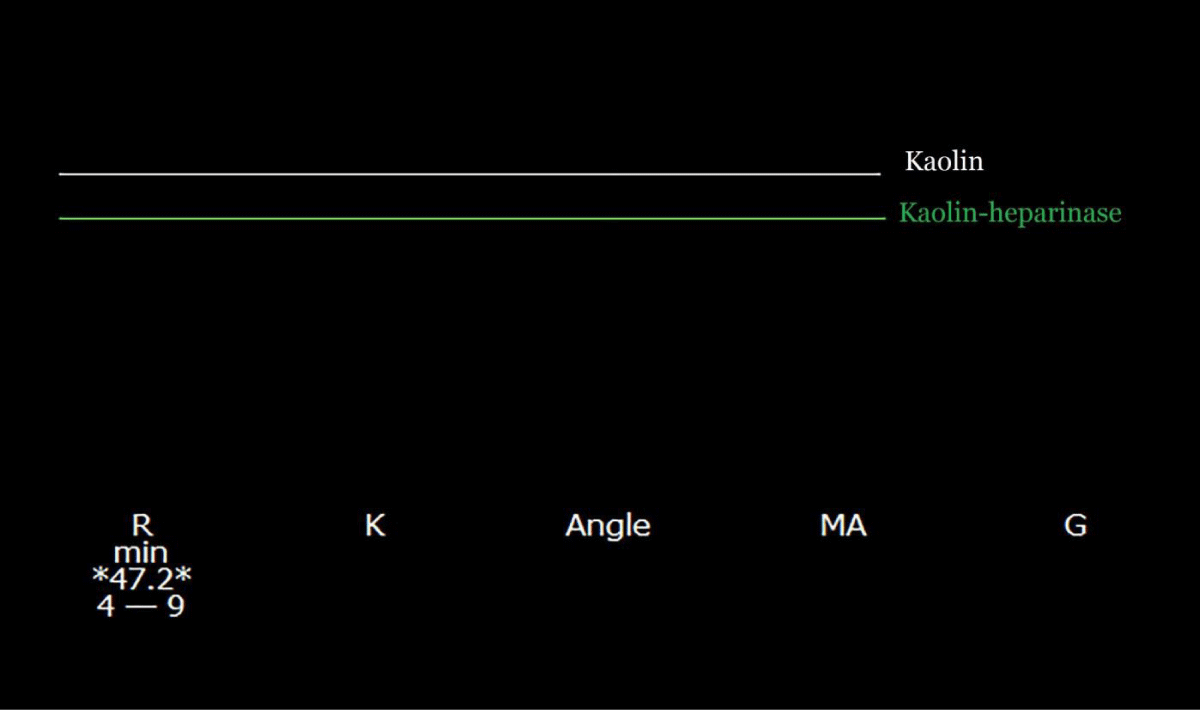
The donor was a young female who died of a drug overdose. Pre-incisional kaolin (K)- activated thromboelastography (TEG) tracing was normal (Figure 1).
Figure 1: A pre-incisional kaolin-activated thromboelastogram. All parameters are within the normal range, suggesting a balance of coagulation between the simultaneously diminished pro-, and anticoagulation drivers.
In this context, a normal tracing signifies a balance of coagulation between the simultaneously diminished pro-, and anticoagulation drivers [12] but should not be misconstrued to suggest normal hemostasis. The corresponding decrease in pro-, and anticoagulation factors, renders hemostasis imbalance-prone [7]. In routine LT cases, no special therapy is usually required. However, prior to procedures with major hemostatic challenges (e.g. redo liver, or encapsulating peritoneal sclerosis), a rational approach would be to simultaneously replenish pro-, and anticoagulation factors, by transfusing fresh frozen plasma (FFP) [13]. The gained increase in hemostatic reserve would lessen the hemostatic volatility during subsequent surgical challenges.
During the pre-anhepatic phase, modest hemorrhage necessitated transfusion of two red blood cell (RBC) units. At the completion of the dissection, the portal vein was clamped.
The anhepatic period is characterized by vascular stasis, secondary to arterial, portal, and caval clamps, and cessation of all hepatic functions. Activation of coagulation during the anhepatic period is due to (1) hemodynamic instability (low flow states) [3,14,15]. (2) excess production and diminished clearance of microparticle-bound tissue factor; (3) vascular stasis; and (4) nitric-oxide mediated platelets’ and coagulation proteins’ dysfunction in response to precipitous portal hypertension [5,16]. Additionally, increased production and diminished clearance of circulating tissue plasminogen activator (tPA) promote fibrinolysis [5]. Activation of fibrinolysis alone results in primary hyperfibrinolysis [17], a non-prothrombotic state with hemorrhagic diathesis, while the simultaneous activation of coagulation and fibrinolysis frequently results in DIC. Intravenous heparin is routinely administered in our institution just prior to portal clamping, for the dual purpose of thromboprophylaxis, and inhibition of consumptive coagulopathy. Its routine use in LT remains, seemingly, underutilized, and only sporadically reported [7,10,18]. Heparin effect and the underlying hemostasis should be assessed by K-TEG and kaolin-heparinase (KH)-TEG or similar VEM. For adequate heparinization, the R-time ratio of K/KH TEG tracings should be 3-5.
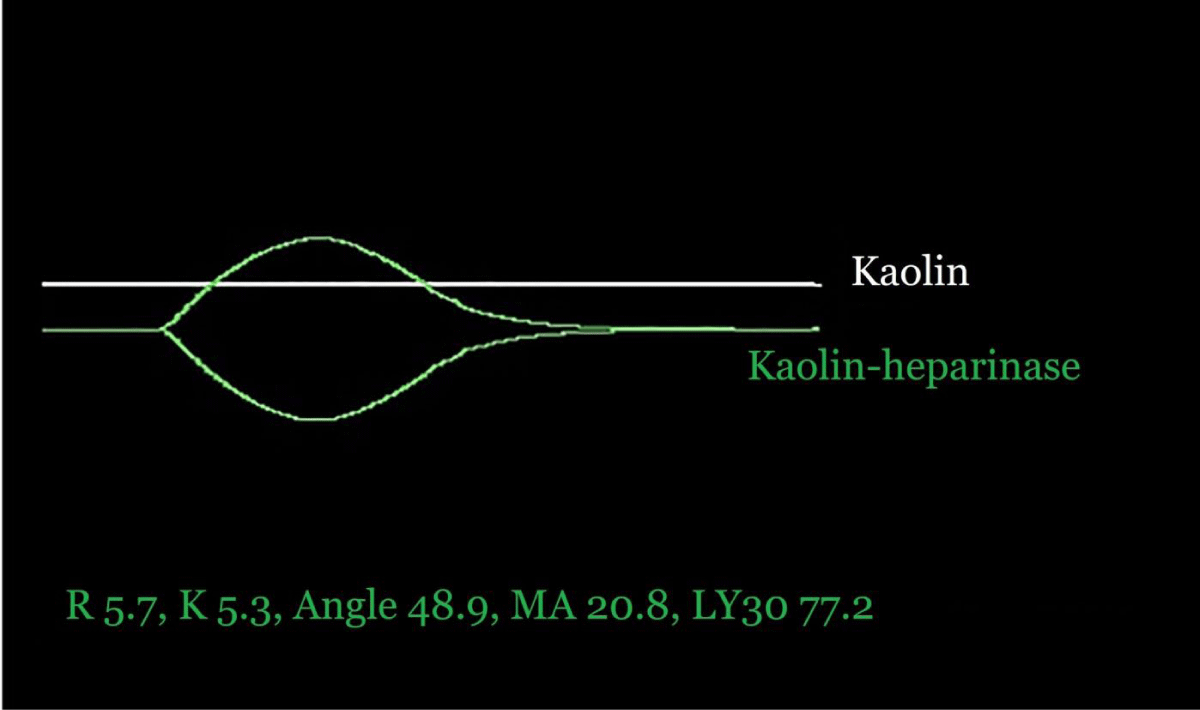
Figure 2 shows TEG tracings 10 min into the anhepatic phase. A 30 u/kg prophylactic heparin bolus resulted in a “flat-line” K-TEG tracing, while KH-TEG revealed the underlying hyperfibrinolysis [19,20].Figure 2: Kaolin and kaolin-heparinase thromboelastograms were drawn 10 min after portal clamping (anhepatic phase). A 30 u/kg prophylactic heparin bolus was given shortly prior to portal clamping, and resulted in a “flat-line” kaolin tracing, while the kaolin-heparinase tracing displays severe hyperfibrinolysis.
It has been suggested that in primary hyperfibrinolysis the TEG R-time (or ROTEM CT) is normal, in contrast to a short R-time in Type 1 DIC [19,21]. A scientific validation has never been provided, and recent evidence suggests otherwise [22-24]. The transplant anesthesiologist should, therefore, cautiously presume that all VEM assays displaying hyperfibrinolysis are suggestive of prothrombotic proclivity.
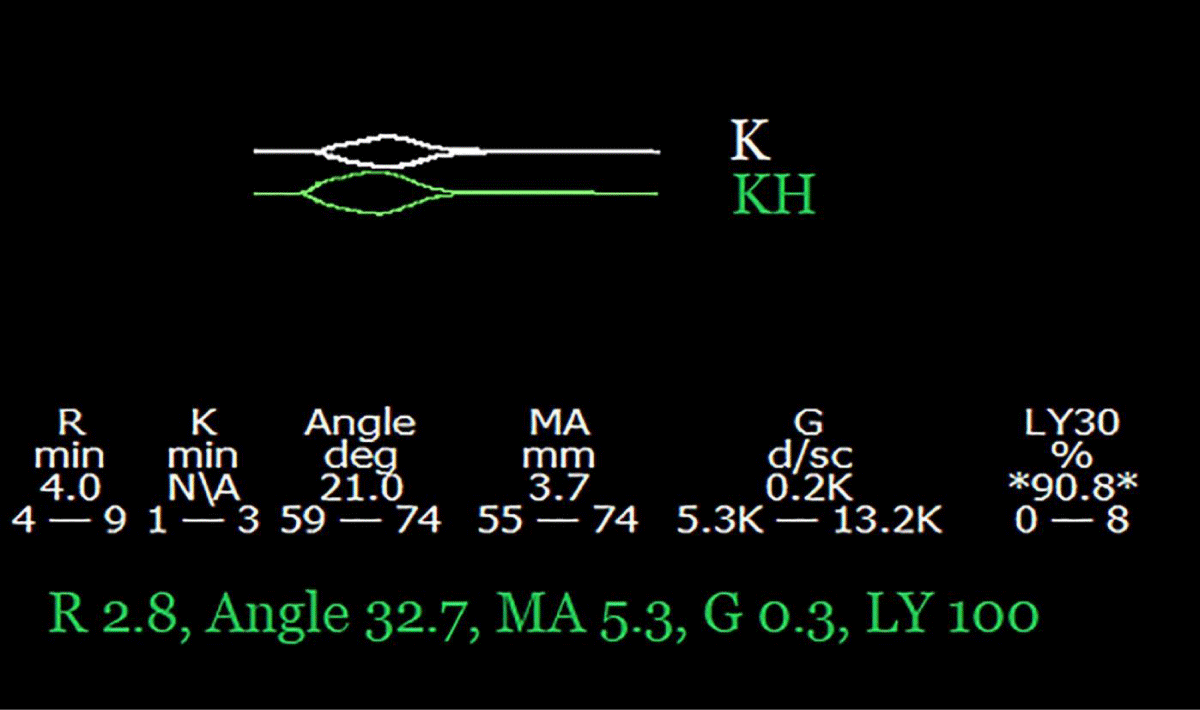
No thrombus was found within the transected portal vein, but the portal flow was severely reduced, secondary to long-standing portal thrombosis. The portal flow increased considerably after ligation of several large portomesenteric-systemic collaterals, but the procedure prolonged the anhepatic phase and caused significant hemorrhage that necessitated the transfusion of 14 units RBC and 14 units FFP. TEG drawn 60 min later, while still anhepatic, shows worsening hyperfibrinolysis, a short R-time, and a lack of heparin effect (Figure 3).
Figure 3: Kaolin and kaolin-heparinase thromboelastograms were drawn 70 min after portal clamping (anhepatic phase) and during significant hemorrhage. The combination of hyperfibrinolysis, with a short R-time, suggests disseminated intravascular coagulation (DIC). The short R-time on the kaolin tracing indicates a lack of heparin effect.
Shortening R time on TEG suggests activation of coagulation [25] and strongly supports the notion that the hyperfibrinolysis is secondary to DIC. The loss of heparin effect is due to a combination of short half-life, significant blood loss, and an unusually long anhepatic phase. To maintain adequate heparinization while in DIC, the anesthesiologist should be mindful to reduce heparin during significant blood loss, and monitor its effect frequently. In the absence of effective anticoagulation, the recipient becomes thrombosis-prone.
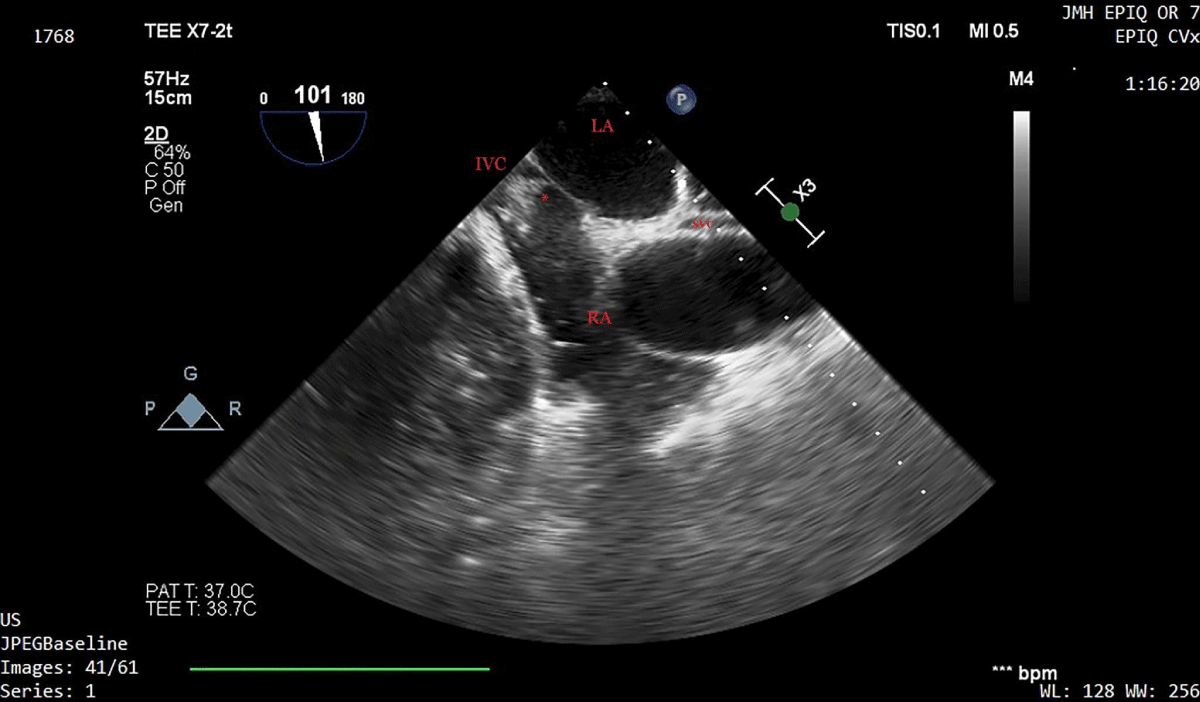
Ten minutes later, a sudden and severe hypotensive episode occurred, and a large thrombus was seen at the confluence of the inferior vena cava and the right atrium, on a two-dimensional transesophageal echocardiographic bicaval view (Figure 4 and Supplementary Video 1). Vasopressors and 2000 units of heparin were promptly given, and hemodynamic stability was fully restored within 5 minutes.
Figure 4: Two-dimensional transesophageal echocardiographic bicaval view during a severe hypotensive episode, 10 minutes prior to reperfusion. A large thrombus is seen at the confluence of the inferior vena cava and the right atrium. Heparin 2000 units and vasopressors were administered and hemodynamic stability was restored. The clot dispersed over 10 minutes. LA: Left Atrium; RA: Right Atrium; IVC: Inferior Vena Cava; SVC: Superior Vena Cava; *Intracardiac clot.
Video 1: Two-dimensional transesophageal echocardiographic bicaval view during a severe hypotensive episode, 10 minutes prior to reperfusion. A large thrombus is seen at the confluence of the inferior vena cava and the right atrium. Heparin 2000 units and vasopressors were administered and hemodynamic stability was restored. The clot dispersed over 10 minutes. (https://youtu.be/vG4_mWMk658).
Intracardiac thrombosis and/or pulmonary embolism are well-described and dreaded complications of LT and are frequently associated with poor outcome [3,26]. Prompt diagnosis and therapy are paramount. Heparin (2000-10,000 units) is nearly always needed to prevent clot propagation. Recombinant t-PA (0.5-4 mg) should be considered for severe, vasopressors-resistant hemodynamic instability [3,26].
Shortly thereafter, the graft was reperfused uneventfully. Five minutes into the neohepatic phase, K-, and KH-TEG displayed a “flat-line” (Figure 5).
Figure 5: Kaolin and kaolin-heparinase thromboelastograms were drawn 5 minutes after reperfusion. “Flat-line” tracings suggest extreme primary or secondary hyperfibrinolysis (a DIC variant). Blood products (FFP, platelets, and cryoprecipitate) along with an epsilon-aminocaproic acid infusion of 1 gr/h were administered.
The early neoheaptic period is fraught with hemostatic derangements secondary to: (1) heparin, or tPA (DCD donor) given to the donor during procurement [27]; (2) release of heparinoid substances [27,28], thrombomodulin [27], or tPA from the allograft [27,28]; (3) an increase in circulating Von Willebrand factor [5]; and (4) dysfunctional endothelium of the hepatic allograft [7]. The differential diagnosis of a “flat-line” tracing includes heparin or heparinoids effect, exogenous or endogenous tPA effect (i.e primary fibrinolysis) [17], severe DIC [20], and extreme dilutional or consumptive factors deficiency. Extreme factors deficiency is rare and usually self-evident from the clinical context. In the early neohepatic period, the distinction between primary hyperfibrinolysis and DIC as the etiology of a new-onset “flat-line” KH-TEG tracing is, at times, difficult, but was apparent in this case in light of preceding DIC during the anhepatic phase.
The surgeon complained of diffuse and significant oozing and a lack of blood clots in the surgical field. Twenty-seven units RBC, 19 units FFP, 6 units platelets, and 5 units cryoprecipitate were administered, and epsilon-aminocaproic acid (Amicar) infusion of 1 gr/h was started with partial improvement of the oozing.
The routine use of antifibrinolytics in LT remains controversial [6]. Antifibrinolytics are contraindicated in DIC, and serious thrombotic events were reported following their use in LT [6]. Nevertheless, antifibrinolytics should be considered when significant hemorrhage is secondary to VEM-proven hyperfibrinolysis. We pretreat with 30 U/kg of heparin all clinically important hyperfibrinolysis or “flat-line” tracings on KH- TEG, prior to the administration of an antifibrinolytic [7,29]. Additionally, we limit the antifibrinolytic therapy to the new hepatic phase, since DIC usually subsides within one hour in the presence of a functioning newly-grafted liver. Following intravascular thrombosis, antifibrinolytics should be deferred until hemodynamic stability is fully restored, and echocardiography confirms complete dissolution or dispersal of the clot.
One hour post-reperfusion, the TEG displayed a mild residual heparin effect, and a 5 mg dose of protamine was administered. Hemostasis gradually returned to normal over the following 4 hours and the procedure was completed. The patient did not require any postoperative transfusion; he was extubated on POD 1 and discharged home after 8 days.
We refrain from reversing the heparin effect in the first hour of the neohepatic period, as heparin protects from DIC-induced thrombosis. We previously reported a case of intracardiac thrombosis after the early administration of protamine [7]. It’s not uncommon for oozing to linger beyond the normalization of VEM and to gradually subside over the course of several hours as was in this case.
Arguably, the significant decline in transfusion requirements in LT in recent years is largely due to advanced understanding and improved hemostatic management of the so-called “rebalanced” coagulation of cirrhosis. Thrombotic complications, however, remain the Achilles heel of LT and are frequently preceded by major bleeding, in a clinical and viscoelastic setting that mimics DIC. The distinction between primary hyperfibrinolysis and DIC in trauma-induced coagulopathy remains controversial [30], and in LT, distinctive VEM tracings of primary hyperfibrinolysis and DIC are not adequately characterized. The argued association of a short R-time (or ROTEM CT) with Type 1 DIC [19,21] is neither validated nor supported by studies in sepsis-associated DIC [22-24]. Therefore, we administer heparin during prolonged hemodynamic instability (low flow state), clinically important hyperfibrinolysis or DIC, hypercoagulability on VEM, and at the onset of the anhepatic phase. As we previously reported, heparin thromboprophylaxis, given at the time of portal clamping, is not usually associated with a clinically meaningful increase in RBC demand, and its effect usually subsides within one hour [10]. During the anhepatic and early neohepatic phase the graft is connected to the recipient’s circulation. Since vascular anastomoses require minimal dissection, hemorrhage secondary to heparin is unusual in both transplant and vascular surgery. It may be argued, however, that heparin, if given during the dissection or neohepatic phase as a therapy for DIC, may increase RBC demand. We find the latter an acceptable trade-off for thrombosis and/or consumptive coagulopathy. In addition, the heparin effect is temporary, and any lingering effect could be reversed with stated precautions.
A “flatline” tracing is not a rare, or well-characterized VEM finding in LT. Its clinical counterpart includes severe thrombo-hemorrhagic tendency and consumptive coagulopathy. Therefore, we regard it as a variant of DIC and treat it with heparin [5,20,31]. Flat-line VEM pattern is especially common in the new hepatic phase, as it was reported in 27% - 71% of (“native”) TEG taken 5-10 min after liver reperfusion [27,28]. Kim, et al., investigated the etiology of 49 flat-line TEG tracings, by adding protamine and/or tranexamic acid to the TEG samples. Hyperfibrinolysis alone was the cause in 38%, as samples clotted with tranexamic acid but not with protamine, while 57% “flat-line” tracings were due to the combination of heparin effect and hyperfibrinolysis, and the addition of both protamine and tranexamic acid was required for a clot to form [28]. Importantly, patients with a flat-line tracing had lower platelet counts, and fibrinogen levels compared to patients without a flat-line tracing, suggesting that consumptive coagulopathy was underway [5].
We advise against the use of antifibrinolytics prior to reperfusion, even if administered concomitantly with heparin, due to an overlap in the clinical and viscoelastic manifestations of primary hyperfibrinolysis and DIC in LT. In addition, it’s difficult to maintain adequate heparinization during hemorrhage, as this case demonstrates, due to loss of heparin in exsanguinated blood, and subtherapeutic heparin levels in the presence of an antifibrinolytic may result in catastrophic thrombosis.
In conclusion, the case describes the hemostatic perturbations along the various stages of LT and highlights some of the more challenging diagnostic and management decisions in LT. We also present a safe, carefully structured approach to hyperfibrinolysis and its “flat-line” variant. The case also underscores the importance of VEM and TEE in the successful management of LT.
- Gologorsky E, De Wolf AM, Scott V, Aggarwal S, Dishart M, et al. Intracardiac thrombus formation and pulmonary thromboembolism immediately after graft reperfusion in 7 patients undergoing liver transplantation. Liver Transpl. 2001; 7: 783-789. PubMed: https://pubmed.ncbi.nlm.nih.gov/11552212/
- Lerner AB, Sundar E, Mahmood F, Sarge T, Hanto DW, et al. Four cases of cardiopulmonary thromboembolism during liver transplantation without the use of antifibrinolytic drugs. Anesth Analg. 2005; 101: 1608-1612. PubMed: https://pubmed.ncbi.nlm.nih.gov/16301227/
- Sakai T, Matsusaki T, Dai F, Tanaka KA, Donaldson JB, et al. Pulmonary thromboembolism during adult liver transplantation: incidence, clinical presentation, outcome, risk factors, and diagnostic predictors. British J Anaesth. 2012; 108: 469-477. PubMed: https://pubmed.ncbi.nlm.nih.gov/22174347/
- Warnaar N, Molenaar IQ, Colquhoun SD, Slooff MJH, Sherwani S, et al. Intraoperative pulmonary embolism and intracardiac thrombosis complicating liver transplantation: a systematic review. J Thromb Haemost. 2008; 6: 297-302. PubMed: https://pubmed.ncbi.nlm.nih.gov/18005235/
- Blaine KP, Sakai T. Viscoelastic Monitoring to Guide Hemostatic Resuscitation in Liver Transplantation Surgery. Semin Cardiothorac Vasc Anesth. 2018; 22: 150-163. PubMed: https://pubmed.ncbi.nlm.nih.gov/29099334/
- Bezinover D, Dirkmann D, Findlay J, Guta C, Hartmann M, et al. Perioperative Coagulation Management in Liver Transplant Recipients. Transplantation. 2018; 102: 578-592. PubMed: https://pubmed.ncbi.nlm.nih.gov/29337842/
- Raveh Y, Shatz V, Lindsay M, Nicolau-Raducu R. Disseminated intravascular coagulation during liver transplantation unleashed by protamine. J Clin Anesth. 2019; 57: 117-118. PubMed: https://pubmed.ncbi.nlm.nih.gov/30991212/
- Patrassi GM, Sartori MT, Sgarabotto D, Sturniolo G, Boeri G, et al. A DIC-like picture on plasma and ascitic fluid of cirrhotic patients. Res Exp Med. 1988; 188: 351-356. PubMed: https://pubmed.ncbi.nlm.nih.gov/3147501/
- Tang HH, Salem HH, Wood LJ, Dudley FJ. Coagulopathy during ascites reinfusion: prevention by antiplatelet therapy. Gastroenterology. 1992; 102: 1334-1339. PubMed: https://pubmed.ncbi.nlm.nih.gov/1551539/
- Nicolau-Raducu R, Beduschi T, Vianna R, Diez C, Sleem M, et al. Fibrinolysis Shutdown Is Associated With Thrombotic and Hemorrhagic Complications and Poorer Outcomes After Liver Transplantation. Liver Transplantatio. 2019; 25: 380-387. PubMed: https://pubmed.ncbi.nlm.nih.gov/30548128/
- Tripodi A, Primignani M, Chantarangkul V, Dell'Era A, Clerici M, et al. An imbalance of pro- vs anti-coagulation factors in plasma from patients with cirrhosis. Gastroenterology. 2009; 137: 2105-2111. PubMed: https://pubmed.ncbi.nlm.nih.gov/19706293/
- Tripodi A, Mannucci PM. The Coagulopathy of Chronic Liver Disease. New Engl J Med. 2011; 365: 147-156. PubMed: https://pubmed.ncbi.nlm.nih.gov/21751907/
- Drebes A, de Vos M, Gill S, Fosbury E, Mallett S, et al. Prothrombin Complex Concentrates for Coagulopathy in Liver Disease: Single-Center, Clinical Experience in 105 Patients. Hepatol Commun. 2019; 3: 513-524. PubMed: https://pubmed.ncbi.nlm.nih.gov/30976742/
- Lip GY, Gibbs CR. Does heart failure confer a hypercoagulable state? Virchow's triad revisited. J Am Coll Cardiol. 1999; 33: 1424-1426. PubMed: https://pubmed.ncbi.nlm.nih.gov/10193748/
- Rasche H. Haemostasis and thrombosis: an overview. Eur Heart J Suppl. 2001; 3: Q3-Q7.
- Nielsen VG. Nitric oxide decreases coagulation protein function in rabbits as assessed by thromboelastography. Anesth Analg. 2001; 92: 320-323. PubMed: https://pubmed.ncbi.nlm.nih.gov/11159223/
- Hunt BJ, Segal H. Hyperfibrinolysis. J Clini Pathol. 1996; 49: 958. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC499639/
- Nicolau-Raducu R, Occhipinti E, Marshall T, et al. Thromboprophylaxis With Heparin During Orthotopic Liver Transplantation: Comparison of Hepcon HMS Plus and Anti-Xa Assays for Low-Range Heparin. J Cardiothorac Vasc Anesth. 2017; 31: 575-581. PubMed: https://pubmed.ncbi.nlm.nih.gov/27745797/
- Pohlman TH, Fecher AM, Arreola-Garcia C. Optimizing transfusion strategies in damage control resuscitation: current insights. J Blood Med. 2018; 9: 117-133. PubMed: https://pubmed.ncbi.nlm.nih.gov/30154676/
- Raveh Y, Rodriguez Y, Pretto E, Souki F, Shatz V, et al. Thrombotic and hemorrhagic complications during visceral transplantation: risk factors, and association with intraoperative disseminated intravascular coagulation-like thromboelastographic qualities: a single-center retrospective study. Transpl Int. 2018; 31: 1125-1134. PubMed: https://pubmed.ncbi.nlm.nih.gov/29786890/
- Nickson C. Thromboelastogram (TEG). 2019. PubMed: https://litfl.com/thromboelastogram-teg/
- Farkas J. PulmCrit- Coagulation balance in sepsis-associated DIC. 2016. PubMed: https://emcrit.org/pulmcrit/sepsis-dic/
- Müller MCA, Meijers JC, van Meenen DM, Thachil J, Juffermans NP. Thromboelastometry in critically ill patients with disseminated intravascular coagulation. Blood Coagul Fibrinolysis. 2019; 30: 181-187. PubMed: https://pubmed.ncbi.nlm.nih.gov/31157682/
- Sivula M, Pettilä V, Niemi TT, Varpula M, Kuitunen AH. Thromboelastometry in patients with severe sepsis and disseminated intravascular coagulation. Blood Coagulation & Fibrinolysis. 2009; 20: 419-426. PubMed: https://pubmed.ncbi.nlm.nih.gov/19581801/
- Hendriks HGD, Meijer K, de Wolf JTM, Porte RJ, Klompmaker IJ, et al. Effects of recombinant activated factor VII on coagulation measured by thromboelastography in liver transplantation. Blood Coagul Fibrinolysis. 2002; 13: 309-313. PubMed: https://pubmed.ncbi.nlm.nih.gov/12032396/
- Verbeek TA, Stine JG, Saner FH, Bezinover D. Hypercoagulability in End-stage Liver Disease: Review of Epidemiology, Etiology, and Management. Transplant Direct. 2018; 4: e403. PubMed: https://pubmed.ncbi.nlm.nih.gov/30534594/
- Harding SA, Mallett SV, Peachey TD, Cox DJ. Use of heparinase modified thrombelastography in liver transplantation. Br J Anaesth. 1997; 78: 175-179. PubMed: https://pubmed.ncbi.nlm.nih.gov/9068337/
- Kim EH, Song SH, Kim GS, Ko JS, Gwak MS, et al. Evaluation of "flat-line" thromboelastography after reperfusion during liver transplantation. Transpl Proc. 2015; 47: 457-459. PubMed: https://pubmed.ncbi.nlm.nih.gov/25769590/
- Asakura H. Classifying types of disseminated intravascular coagulation: clinical and animal models. J Intensive Care. 2014; 2: 20. PubMed: https://pubmed.ncbi.nlm.nih.gov/25520834/
- Hayakawa M. Pathophysiology of trauma-induced coagulopathy: disseminated intravascular coagulation with the fibrinolytic phenotype. J Intensive Care. 2017; 5: 14. PubMed: https://pubmed.ncbi.nlm.nih.gov/28289544/
- El-Baghdadi MM, Sakai T. Fatal Pulmonary Embolism During Liver Transplantation in a Patient With Fulminant Hepatic Failure: A Diagnostic Challenge of the “Flat-Line” Thromboelastogram. J Cardiothorac Vasc Anesth. 2010; 24: 641-643. PubMed: https://pubmed.ncbi.nlm.nih.gov/19819727/