More Information
Submitted: June 22, 2021 | Approved: July 08, 2021 | Published: July 09, 2021
How to cite this article: Daguet D, Venkataramana SH, Thomas JV, Kodimule SP. AsdamarinTM relieves functional dyspepsia in healthy adults in only 7 days: A randomized, double-blind, placebo-controlled pilot study. Ann Clin Gastroenterol Hepatol. 2021; 5: 018-024.
DOI: 10.29328/journal.acgh.1001028
Copyright License: © 2021 Daguet D, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Ferula asafeotida; Silybum marianum; Functional dyspepsia; Liver-stomach disharmony syndrome
Abbreviations: AE: Adverse Events; EOS: End Of Study; FD: Functional Dyspepsia; GCP: Good Clinical Practice; GDSS: Glasgow Dyspepsia Severity Score; GERD: Gastroesophageal Reflux Disease; GSRS: Gastrointestinal Symptom Rating Scale; HPLC: High Performance Liquid Chromatography; IBS: Irritable Bowel Syndrome; ICH: International Conference on Harmonization; IP: Interventional Product; ITT: Intention To Treat; MEC: Multienzyme Complex; NAFLD: Non-Alcoholic Fatty Liver Disease; NDI-SF: Nepean Dyspepsia Index-Short Form; SAE: Serious Adverse Event; SOP: Standard Operating Procedure; TCM: Traditional Chinese Medicine
AsdamarinTM relieves functional dyspepsia in healthy adults in only 7 days: A randomized, double-blind, placebo-controlled pilot study
David Daguet1* , Sudeep Heggar Venkataramana2, Justin V Thomas3 and Shyam Prasad Kodimule2
, Sudeep Heggar Venkataramana2, Justin V Thomas3 and Shyam Prasad Kodimule2
1Vidya Europe SAS, 6 avenue de la Baltique, 91140 Villebon sur Yvette, France
2Vidya Herbs (P) Ltd., R&D Center for Excellence Jigani Industrial Area, Anekal Taluk, Bangalore, Karnataka, India
3Leads Clinical Research and Bio Services Private Ltd, No 9, Myhthri Legacy Ist Floor Chelekere Main Road Kalyan Nagar, Bangalore, Karnataka, 560043 India
*Address for Correspondence: David Daguet, Vidya Europe SAS, 6 avenue de la Baltique, 91140 Villebon sur Yvette, France, Tel: +33 2 18 56 24 51; Email: [email protected]
Functional dyspepsia (FD) is a prevalent global health concern increasing with years. Inspired by the Traditional Chinese Medicine (TCM) liver-stomach disharmony syndrome in order to find a quick natural alternative treatment, a Ferula asafoetida-Silybum marianum (Asdamarin™) combined extract has been developed and proved its rapid efficiency and its safety with a 7-day randomized, double-blind, placebo-controlled pilot study (CTRI/2018/05/013993 dated 21/05/2018) conducted on 70 healthy human volunteers (aged 18–60 years) supplemented with 250 mg / twice a day of either a placebo or Asdamarin™. Subjects were evaluated from baseline to the end of the study (EOS) through changes in Gastrointestinal Symptom Rating Scale (GSRS), changes in Glasgow Dyspepsia Severity Score (GDSS) and changes in the short form of Nepean Dyspepsia Index (NDI-SF) for Quality of Life. Compared to the baseline a significant reduction (p < 0.001) of GDSS questionnaire score was noted in the Asdamarin™ group (from 5.66 ± 3.1 at baseline to 5.09 ± 2.8 at the End Of Study (EOS)) compared to placebo group (from 2.77 ± 1.3 baseline to 2.69 ± 1.3 EOS), a significant decrease (p < 0.001) of GSRS score noted in the Asdamarin™ group (from 32.11 ± 8.6 baseline to 19.11 ± 5.4 EOS) compared to the placebo group (from 25.23 ± 3.6 baseline to 23.2 ± 4.9 EOS), and a significant reduction (p < 0.001) of NDI-SF scoring was noted in the Asdamarin™ group (from 15.74 ± 4.1 baseline to 11.54 ± 2.1 EOS) compared to placebo group (from 12.54 ± 3.2 baseline to 11.63 ± 2.6 EOS). Asdamarin™ has been found safe and very well tolerated during the study.
Functional dyspepsia is a common digestive trouble also known as indigestion. This specific condition of impaired digestion may include upper abdominal fullness, heartburn, nausea, belching, or upper abdominal pain, and may frequently be caused by gastroesophageal reflux disease (GERD) or gastritis. Functional dyspepsia is also encountered in Irritable Bowel Syndrome (IBS), Non-Alcoholic Fatty Liver Disease (NAFLD), in some diets, associated with food intolerances and as medication’s side effect.
The prevalence of FD was found to be, in 2012, from 23% to 45% when assessed with upper gastrointestinal symptoms inclusion [1]. In 2015 [2], a review investigated FD prevalence and concluded that the overall pooled prevalence was 21% but varying among countries and according to the criteria used to define it.
Medical therapies, natural alternatives like multienzymes complex (MEC) are existing to treat FD. Medical therapies could exert quick different efficiencies but often associated with various types of side effects [3], while MEC is presented by having significant efficiency after no less than 30 days [4]. Then, a natural and quickly efficient alternative could therefore represent the ideal solution.
In TCM, FD is associated, like gastroesophageal reflux disease and chronic gastritis to the liver-stomach disharmony pattern [5]. According to some authors, the liver-stomach disharmony syndrome must be understood as a relationship between these two organs involving the five elements theory of TCM. For another author and complementarily, in TCM, FD is divided into different syndromes in which the basic liver-stomach disharmony syndrome is included [6]. This traditional principle of medicine explains how under abnormal conditions, both the over restriction and the counter-restriction can lead to disharmony between the liver and the stomach.
Based on this principle of TCM, an ingredient (Asdamarin™) has been developed associating two well-known plants dedicated respectively to stomach and liver functions, to treat simultaneously these two organs in order to benefit of a quick relief of FD. The first plant Ferula asafeotida, Umbellifera family, known in Ayurveda as Hing, is a traditional Indian plant used to help digestion. But this plant is also recognized for other activities like renal protection, hypolipidemic, antiatherosclerosis, cardio protection, treatment of insulin resistance and cognition improvement [7]. Asafoetida in combinational herbal preparations has been studied extensively for stomach related ailments and showed, when encapsulated in turmeric nanofibers for example, positive effects by attenuating the disease activity in a rat model of ulcerative colitis [8]. In a rat model of ethanol-induced ulcer a combination of asafoetida and fenugreek fibers exhibited significant stomach protection [9]. Further, in vitro experiment on guinea pig and rat isolated ileums demonstrated the antispasmodic activity of asafoetida [10].
The second plant is Silybum marianum, Asteriaceae family, known as milk thistle and very well-known in Europe for its hepatoprotective activity. The plant is used extensively for treating liver disorders due to its potential antioxidant and hepatoprotective effects. Milk thistle has also been studied for hypoglycemic and antidiabetic activities [11]. The pharmacological benefits of milk thistle have been largely studied in various experimental models, like for anti-inflammatory effects on rats with NASH [12]. A high fat diet-fed rats treated with silybin for 6-weeks, the active principle of milk thistle, exhibited a significant amelioration of non-alcoholic fatty liver disease (NAFLD) [13]. By the combination of extracts of these two plants (Asdamarin™), a natural treatment of FD by simultaneous action on liver and stomach has been attempted. Previously, we have demonstrated the efficacy of Asdamarin™ in improving the gastric emptying alongside the safety assessment in rats [14]. These data and the functional attributes of the two source plants prompted us to validate its effectiveness in a short time treatment pilot clinical trial. The short time treatment challenge having been decided to comply with patient’s expectations of short time relief required by this digestive trouble.
Ingredient composition
The ingredient is composed of 25% of Ferula asafoetida oleoresin obtained by supercritical fluid extraction, and 75% of Silybum marianum hydroalcoholic extract. Asdamarin™ is standardized to 25% of silymarin (HPLC method).
Study design
This was a randomized, double blind, placebo controlled, parallel group pilot study performed on 70 healthy volunteers. This study was performed according to the protocol and standard operating procedures (SOPs) that meet the guidelines laid down by the International Conference on Harmonization (ICH) for Good Clinical Practice (GCP) in clinical trials and in compliance with ethical principles that have their origin in the Declaration of Helsinki, and with the local regulations. The protocol complied with recommendations of the 18th World Health Congress (Helsinki, 1964) and all applicable amendments. The protocol also complied with Indian GCP and Schedule Y (amended version 2013) and Indian Council of Medical Research codes and guidelines.
Ethical considerations
The study protocol and informed consent form were approved by institutional ethics committee (LCBS-VH-33, Aman Hospital and Research Center, Vadodara, Gujarat, India). This clinical study was registered prospectively in Clinical Trials Registry – India (CTRI/2018/05/013993 dated 21/05/2018). Informed consent and consent to publish were obtained prior to subject randomization. Subjects meeting all inclusion and no exclusion criteria signed a written informed consent and were enrolled in the study.
Determination of sample size
The sample size calculation was based on difference b/w results of Baseline and EOS of the subjects are considered medically relevant. Assuming a common SD of 2.8 at the end of treatment, 30 subjects would be sufficient to detect a difference of 1.5 in mean difference b/w the Baseline and EOS with power of 80% and a 0.05. 2-sided level of significance.
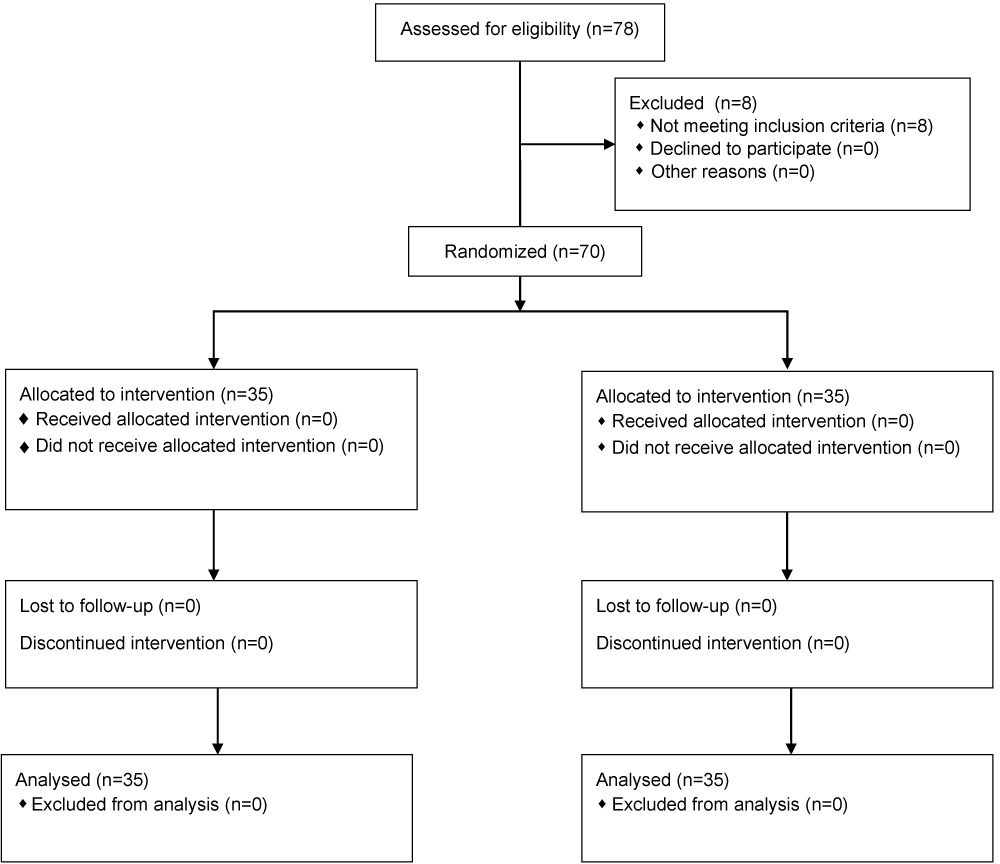
A sample size of 62 subjects, 31 in each arm, was considered sufficient to detect a clinically important difference between groups with 80% power and a 5% level of significance. Considering a dropout rate of 10% the sample size was finalized as 70 after 78 people were screened. Subjects were randomized using computer randomization with 35 subjects per group, with the demographic characteristics presented in table 1. GSRS, GDSS and NDI-SF scores were classified into domains/scales. Score and its change from baseline were summarized by treatment groups and visit. Group wise comparison of the mean scores between treatment groups was done by using t-test with 5% level of significance. All the participants received the treatment and were analyzed.
| Table 1: Demographics Characteristics (Gender, height, weight, and BMI) of Subjects Enrolled. | ||||||
| AsdamarinTM (N=35) | Placebo (N=35) | All (N=70) | ||||
| Gender | Number | % | Number | % | Number | % |
| Male | 24 | 68.57 | 16 | 45.71 | 40 | 57.14 |
| Female | 11 | 31.43 | 19 | 54.29 | 30 | 42.86 |
| Height Mean (cm) | 164.90 ± 6.67 | 162.50 ± 5.74 | 163.69 ± 6.30 | |||
| Weight Mean (kg) | 61.34 ± 9.06 | 56.74 ± 9.29 | 59.04 ± 9.40 | |||
| BMI Mean (kg/m2) | 22.63 ± 3.70 | 21.46 ± 3.08 | 22.04 ± 3.43 | |||
A total of N number of subjects required in the study with all the data being complete for analysis, but a proportion (q) are expected to drop out before the study ends. In this case, the following total number of subjects (N1) would have to be enrolled to ensure that the final sample size (N) in each Treatment group is:
Where q is the proportion of attrition and is generally 15% in this type of studies.
Note: The proportion of eligible subjects who will refuse to participate (drop out) or provide the inadequate information will be unknown at the beginning of the study. Approximate estimates are often possible using information from similar studies.
Participants
The subjects were recruited by newspapers adver-tisements and received financial compensation. They all underwent an initial screening evaluation for selection meeting all inclusion and exclusion criteria.
The inclusion criteria were male and female subjects, 18-60 years (both inclusive), fulfilling Rome III diagnostic criteria for functional dyspepsia, who were able to give informed consent and comply with the study procedures, female subjects who were non-pregnant, non-lactating, postmenopausal, surgically sterilized, or using a medically acceptable form of birth control, as determined by the investigator. Recruited Subjects will be suffering from upper abdominal pain or discomfort (i.e. a negative feeling in the upper abdomen that did not reach the level of pain and was characterized by one or more symptoms, including early satiety, postprandial fullness, bloating or nausea) for at least 3 months without an identifiable structural or biochemical abnormality. No organic findings following upper GI endoscopy, abdominal sonography (both in preceding 6 weeks), and routine blood count and blood chemistry analysis. This kind of subjects are considered as healthy because only suffering of functional troubles without any other organic or physiological diseases, no history of peptic ulcer, gastro esophageal reflux disease [15], gastro intestinal surgery or any other clinically significant gastro intestinal disease, gastrointestinal bleeding, mechanical obstruction, perforation or gastrointestinal cancer [16].
The exclusion criteria were: history of peptic ulcer, gastroesophageal reflux disease, gastrointestinal surgery or any other clinically significant gastrointestinal disease, gastrointestinal bleeding, mechanical obstruction, perforation, gastrointestinal cancer; subjects who had taken antibiotics or any other drugs in last 2 weeks with primary site of action in the gastrointestinal tract, history of psychiatric illness, history of congestive heart failure or uncontrolled hypertension, subjects with abnormal hematological or biochemical parameters, pregnant or lactating women or those planning for conception, any additional condition(s) that in the Investigators opinion warranted exclusion from the study or prevent the subject from completing the study.
Randomization and blinding
Selected subjects were randomized (computer generated randomization) into 2 treatment arms to receive Asdamarin™ or placebo in 50:50 ratio. Each randomized subject received a xx-digit randomization number. Randomized subjects who terminated their study participation for any reason, regardless of whether the IP was taken or not, retained their randomization number.
Intervention and outcomes
The consecutive enrollees were divided in two groups: verum and placebo (Figure 1). Verum group was assigned to take orally 250 mg of Asdamarin™ plus 150 mg maltodextrin twice a day in morning and night after food for 7 days, while placebo group was assigned to take orally 400 mg of maltodextrin twice a day in morning and night after food for 7 days. Verum and placebo capsules were provided by Vidya Herbs Pvt Ltd (India). Verum and placebo capsules had similar aspect, odor, and taste.
Figure 1: Study flowchart.
Each subject has been evaluated from baseline to end of the study through changes in Gastrointestinal Symptom Rating Scale (GSRS), changes in Glasgow Dyspepsia Severity Score (GDSS) and changes in the short form of Nepean Dyspepsia Index (NDI-SF) for Quality of Life. The safety of the interventional product has been assessed through physical examination results, incidence of abnormal vital signs, clinically significant changes in laboratory parameters (Aspartate aminotransferase, Alanine aminotransferase, Alkaline phosphatase, Complete blood count, Serum creatinine) and incidence of adverse events (AE) and serious adverse events (SAE) reported.
Statistical analysis
GSRS, GDSS and NDI-SF scores were classified into domains/scales. Score and its change from baseline were summarized by treatment groups and visit. GroupWise comparison of the mean scores between treatment groups was done by using t-test with 5% level of significance.
Subjects are included in the Per Protocol population if they complete the study treatment without any major protocol deviations. Subjects are included in the ITT population if they have taken at least one day of IP and have at least one post baseline assessment. GraphPad Prism version 9.0 has been used for the statistical analysis.
Gastrointestinal symptom scale scorem
The gastrointestinal symptom scale (GSRS) is a Swedish, disease-specific and self-administered questionnaire, designed to evaluate perceived severity of gastrointestinal symptoms during the previous week. It includes 15 items and use a 7-grade Likert scale. The items are divided into five dimensions representing reflux syndrome (two items), abdominal pain syndrome (three items), constipation syndrome (three items), indigestion syndrome (four items) and diarrhea syndrome (three items). Subjects have been evaluated at two visits, at the beginning and at the end of the study.
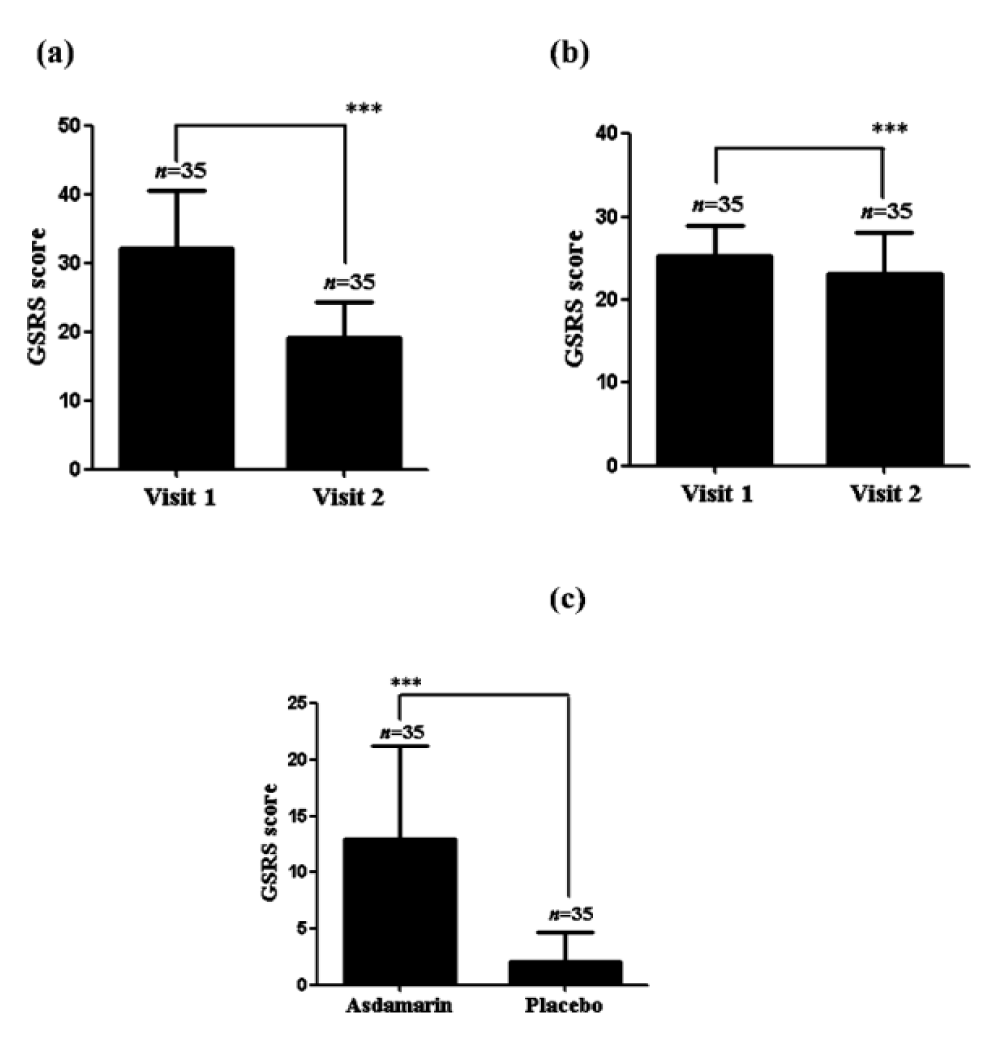
There was a significant decrease (p < 0.001) of GSRS score noted in the Asdamarin™ group (from 32.11 ± 8.6 at baseline to 19.11 ± 5.4 at end of study (EOS)) compared to the placebo group (from 25.23 ± 3.6 at baseline to 23.2 ± 4.9 at EOS) (Figure 2 a,b). A highly significant (p < 0.001) mean change from baseline in the GSRS score was observed in the Asdamarin™ group (13.0 ± 8.3) compared to placebo group (2.0 ± 2.7) at the end of the study (Figure 2c).
Figure 2: GSRS evolution in 7 days, (a) Asdamarin™, (b) placebo and changes from baseline (c); n = 35 subjects per group, ***p < 0.001. The existing study data were analyzed using non-parametric paired t test for comparing the scores within the group and non-parametric independent t-test between the groups..
Glasgow dyspepsia severity score
Glasgow dyspepsia severity score (GDSS) is a validated multidimensional disease specific scale for dyspepsia. It focuses on several aspects of dyspepsia: firstly, the frequency of dyspepsia symptoms and the effect that they have on normal activities and ability to work; secondly, the need for consultations with physicians for dyspepsia and the need for diagnostic investigations for dyspepsia; and thirdly, the need for over the counter and prescription medication for dyspepsia. Subjects have been evaluated at two visits, at the beginning and at the end of the study.
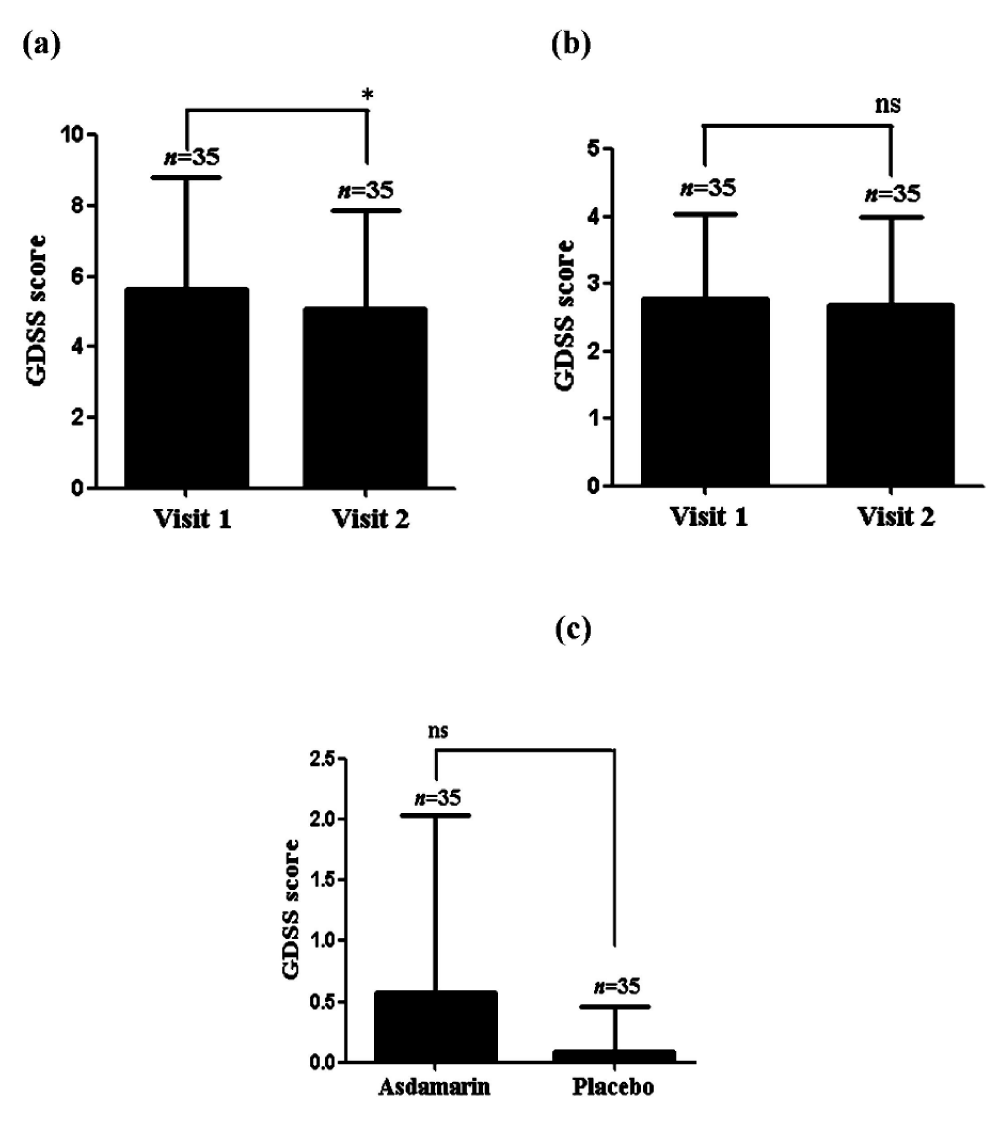
Compared to the baseline a significant reduction (p < 0.001) of GDSS questionnaire score was noted in the Asdamarin™ group (from 5.66 ± 3.1 at baseline to 5.09 ± 2.8 at EOS) compared to placebo group (from 2.77 ± 1.3 at baseline to 2.69 ± 1.3 at EOS) (Figure 3 a,b). A significant (p < 0.05) mean change from baseline in the GDSS questionnaire score was observed in the Asdamarin™ group (0.57 ± 1.7) compared to placebo group (0.08 ± 0.4) at the end of the study (Figure 3c).
Figure 3: GDSS evolution in 7 days (a) Asdamarin™, (b) placebo and changes from baseline (c) n = 35 subjects per group, *p < 0.05, ns: not significant. The existing study data were analyzed using non-parametric paired t test for comparing the scores within the group and non-parametric independent t test between the groups.
Nepean dyspepsia index
The short form of Nepean Dyspepsia Index (NDI-SF) is reliable and valid measure of quality of life in non-ulcer functional dyspepsia. NDI-SF consists of 10 questions with 5 domains (tension, interference with daily activity, eating/drinking, knowledge/control, work/study). Subjects have been evaluated at two visits, at the beginning and at the end of the study.
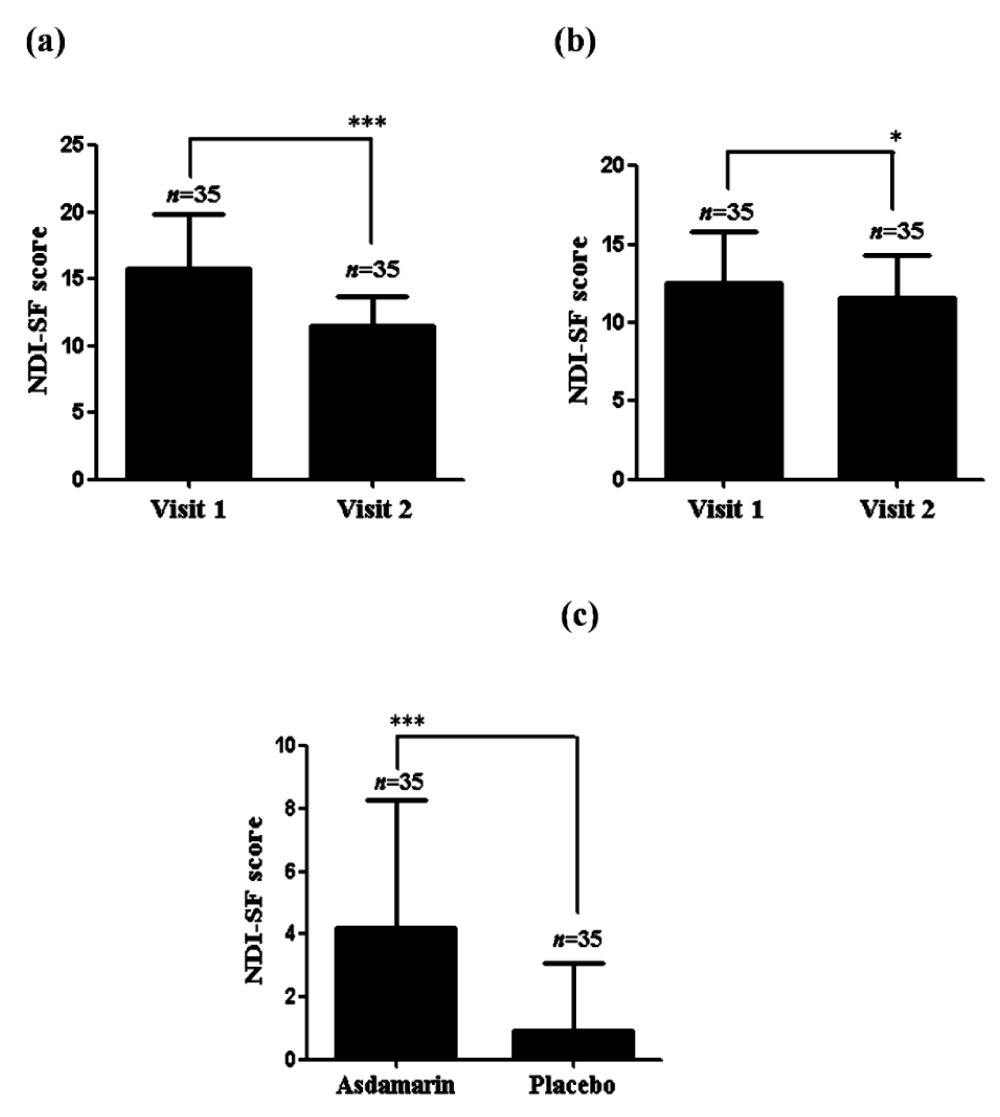
Compared to the baseline a significant reduction (p < 0.001) of NDI-SF scoring was noted in the Asdamarin™ group (from 15.74 ± 4.1 at baseline to 11.54 ± 2.1 at EOS) compared to placebo group (from 12.54 ± 3.2 at baseline to 11.63 ± 2.6 at EOS) (Figure 4 a,b). A significant (p < 0.001) mean change from baseline in the NDI-SF scoring was observed in the Asdamarin™ group (4.2 ± 4.08) compared to placebo group (0.91 ± 2.2) at the end of the study (Figure 4c).
Figure 4: NDI-SF evolution in 7 days (a) Asdamarin™, (b) placebo and changes from baseline (c); n = 35 subjects per group, ***p < 0.001, *p < 0.05. The existing study data were analyzed using non-parametric paired t test for comparing the scores within the group and non-parametric independent t test between the groups.
Analysis of individual domains of NDI-SF quality of life aspects showed marked improvement in tension, interference with daily activities and eating/drinking in the subjects of Asdamarin™ group at the end of study compared to the baseline.
Safety assessment
All seventy subjects who were randomized took the interventional product or the placebo during the complete course of the study. No clinically significant changes were observed in the physical examination during the study in both groups. Throughout the study the vital signs were within the normal level in the subjects of either group. The clinical chemistry and complete blood count parameters were within the normal range and no clinically significant effect was detected in the parameters. Neither AE nor SAEs were reported in the study. Based on the above results, Asdamarin™ 250 mg twice a day was found to be efficacious in reducing the severity of the dyspepsia symptoms, in improving the quality of life, and was found safe and very well tolerated by subjects with non-ulcer functional dyspepsia during this 7-day trial. The total absence of AE or SAEs is not surprising due to the known safety of Ferula asafoetida and Silybum marianum usage in food or phytotherapy and because subjects have been selected with non-ulcer functional Dyspepsia. Maltodextrin used for the placebo is without any concern.
The results of the 7-day double-blind controlled pilot study on healthy volunteers ingesting 250 mg twice a day Asdamarin™ versus placebo (maltodextrin) showed significant reduction of FD severity symptoms and increase of quality of life. Consumption of Asdamarin™ also caused no adverse events and was found safe and very well tolerated.
A healthy liver is necessary for the maintenance of a good health because this organ has many important functions, including helping to digest food and process and distribute nutrients. Many conditions including obesity, alcohol abuses, can contribute to the development of chronic liver diseases like cirrhosis, non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and conditions characterized by inflammation and liver cells damage. FD being a shared symptom of a lot these liver diseases, like it is also a symptom for gastric ulcer, IBS and IBD.
One hypothesis that can be formulated concerning the physiologically established pertinence of the TCM liver-stomach disharmony syndrome is based on recent evolution dealing with liver and gut health. Inflammatory liver diseases like cirrhosis are known to have an impact on stomach function by decreasing the gastric emptying and explaining the gastrointestinal symptoms of this type of diseases [17,18]. Another important aspect of this kind of liver diseases, is that changes in gut’s microbiota can affect the progression of liver disease [19].
It is explained by the overgrowth of intestinal bacteria that may be induced by liver disease, itself promoted by the blockage of the stomach acid. In liver cirrhosis, it has been demonstrated that gut dysfunctions are common and are associated with gastrointestinal symptoms related to delayed gastric emptying and small bowel transit. These disturbances being related to small bowel bacteria overgrowth and altered intestinal barrier function [20]. Based on this, probiotics have been then presented as potential natural hepato-protecting ingredients in liver diseases [21].
According to the above physiologic facts, the hypothesis that could be stated is a close relationship between stomach and liver functions. Close relationship that in bad conditions could be considered as a potential vicious circle in which altered liver function, or liver inflammation, may lead to a reduction of gastric emptying, that may in return, favorize liver disease. This hypothesis of this vicious circle can be a representation of the TCM liver-stomach disharmony. But as described above, liver disease may also be promoted by intestinal bacteria overgrowth that may contribute, in return, to this organ inflammation and then maintains this vicious circle with gut disturbance as a third contributor. This illustrated by that one of the major symptoms associated to cirrhosis is an altered gut transit due to intestinal barrier disfunction promoted by small bowel bacteria overgrowth.
The particular relationship and interdependence between the liver, the stomach and the gut, considered through scientific studies on liver and stomach related diseases, could be contemplated as a modern representation of the TCM liver-stomach harmony principle, enriched with the gut interaction. Of course, in case of diseases or digestive troubles, it could be considered as a modern representation of the TCM liver-stomach disharmony syndrome, a liver-stomach-bowel disharmony.
Rats treated with the respective doses of Asdamarin™ exhibited significant increased percentage 47.97 ± 3.21% of gastric emptying as compared to control 9.07 ± 15.62% and Domperidone 65.77 ± 8.67% used as standard reference. Further, in this study the gastrointestinal transit (GIT) was found to be increasing significantly in the extract and standard drug treated rats as compared to control. The increase in GIT was 59.99% in animals treated with single dose of Domperidone while the extract administered rats showed significant improvement in the GIT by respectively 68, 66.54 and 70.28 for 10, 25 and 50 mg/kg of extract [14].
This pilot study, with its limited means, could be considered as a demonstration of the pertinence of the TCM liver-stomach harmony principle. By treating simultaneously, the stomach with asafetida and the liver with milk thistle, the above-described vicious circle has been breached. The association of the asafetida influence on food transit time [22] and the liver health benefits of milk thistle [23] came out to be a natural prokinetic agent. By improving gastric emptying, gastrointestinal transit time and by regenerating liver intrinsic capacities, the stomach and the liver were treated simultaneously in a way that permit to relieve functional dyspepsia in only 7 days, when other studies with other asafetida formulation without liver active ingredient, obtained significant relief in 30 days [24].
This study could be considered as a starting point for further experiments due to its limitations, mainly a very short duration and the FD mild severity of the subjects recruited. The results obtained, with statistical significance on mild severity FD subjects, are encouraging because it must be considered more difficult to obtain significant differences in mild severity scoring FD during a so short trial. The ingredient health benefit is worthy of interest and may deserve more experimentations on longer durations, on more severe FD, with or without comparison with medical or natural treatments. This study has been conducted on healthy subject, without digestive diseases or troubles characterized by FD, and it could be very instructive to carry out such experiments on NAFLD or IBS people.
In summary, it has been found that the consumption of Asdamarin™ 250 mg twice daily significantly relieve mild functional dyspepsia in only 7 days. The short duration of this study was a challenge. But this challenge was necessary to assess the quick efficiency reached by people suffering of functional dyspepsia. By effectively and simultaneously treating the stomach and the liver, we confirmed that considering under physiological aspects the ancient TCM principle of liver-stomach disharmony syndrome, could be fruitful.
Further studies on the long-term effect of Asdamarin™, with shorter time visit, on other populations like IBS, NAFLD, NASH for example, with monitoring on stomach, liver, but also intestine functions, could be very instructive on how these three organs interact in different health troubles that have functional dyspepsia in common symptom. And of course, to increase our knowledge and comprehension on the mechanism of action of this ingredient.
The authors would like to thank all the participants who take part in this study, along with the researchers and staff of Aman Hospital & Research Center and Leads Clinical Research and Bio Services Private Limited who directed the pilot study.
Funding: This research was funded by Vidya Herbs (P) Ltd, India
Conflicts of interest: DD, HVS and KSP are employees of Vidya Herbs group that funded the pilot study (carried out by a third Contract Research Organization, Leads Clinical Research and Bio Services Private Limited).
- Kumar A, Pate J, Sawant P. Epidemiology of functional dyspepsia. J Assoc Physicians India. 2012; 60: 9-12. PubMed: https://pubmed.ncbi.nlm.nih.gov/23155797/
- Ford AC, Marwaha A, Sood R, Moayyedi P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut. 2015; 64: 1049-57. PubMed: https://pubmed.ncbi.nlm.nih.gov/25147201/
- Talley N. Functional Dyspepsia: Advances in Diagnosis and Therapy. Gut Liver. 2017; 11: 349-357. PubMed: https://pubmed.ncbi.nlm.nih.gov/28452210/
- Majeed M, Majeed S, Nagabhushanam K, Arumugam S, Pande A, et al. Evaluation of the Safety and Efficacy of a Multienzyme Complex in Patients with Functional Dyspepsia: A Randomized, Double-Blind, Placebo-Controlled Study. J Med Food. 2018; 21: 1120-1128. PubMed: https://pubmed.ncbi.nlm.nih.gov/30156436/
- Zhao L, Wang T, Dong J, Chen A, Li G. Liver-stomach disharmony pattern: theoretical basis, identification and treatment. Tradit Chin Med Sci. 2018; 5: 53-57. Sciencedirect: https://www.sciencedirect.com/science/article/pii/S2095754818300085
- Wang C, Zhu M, Xia W, Jiang W, Li Y. Meta-analysis of Traditional Chinese Medicine in treating functional dyspepsia of liver-stomach disharmony syndrome, J Trad Chin Med. 2012; 32: 515-522. PubMed: https://pubmed.ncbi.nlm.nih.gov/23427381/
- Amalraj A, Gopi S. Biological activities and medicinal properties of Asafoetida: A review. J Tradit Complement Med. 2016; 7: 347-359. PubMed: https://pubmed.ncbi.nlm.nih.gov/28725631/
- Gopi S, Amalraj A, Jude S, Varma K, Sreeraj TR, et al. Preparation, characterization and anti-colitis activity of curcumin-asafoetida complex encapsulated in turmeric nanofiber. Mater Sci Eng C Mater Biol Appl. 2017; 81: 20-31. PubMed: https://pubmed.ncbi.nlm.nih.gov/28887965/
- Vijayasteltar L, Jismy IJ, Joseph A, Maliakel B, Kuttan R, et al Beyond the flavor: A green formulation of Ferula asafetida oleo-gum-resin with fenugreek dietary fibre and its gut health potential. Toxicol Rep. 2017; 4: 382-390. PubMed: https://pubmed.ncbi.nlm.nih.gov/28959663/
- Bagheri SM, Hejazian SH, Dashti RMH. The relaxant effect of seed’s essential oil and oleo-gum-resin of Ferula Assa-foetida on isolated rat’s ileum. Ann Med Health Sci Res. 2014; 4: 238-241. PubMed: https://pubmed.ncbi.nlm.nih.gov/24761245/
- Khazim K, Gorin Y, Cavaglieri RC, Abboud HE, Fanti P. The antioxidant silybin prevents high glucose-induced oxidative stress and podocyte injury in vitro and in vivo. Am J Physiol Renal Physiol. 2013; 305: F691–F700. PubMed: https://pubmed.ncbi.nlm.nih.gov/23804455/ et al
- Aghazadeh S, Amini R, Yazdanparast R, Ghaffari SH. Anti-apoptotic and anti-inflammatory effects of Silybum marianum in treatment of experimental steatohepatitis. Exp Toxicol Pathol. 2011; 63: 569-74. PubMed: https://pubmed.ncbi.nlm.nih.gov/20471811/
- Yao J, Zhi M, Minhu C. Effect of silybin on high-fat-induced fatty liver in rats. Braz J Med Biol Res. 2011; 44: 652-659. PubMed: https://pubmed.ncbi.nlm.nih.gov/21755261/
- Illuri R, Venkataramana SH, Daguet D, Kodimule SP. Sub-acute and acute toxicity of Ferula asafoetida and Silybum marianum formulation and effect of the formulation on delaying gastric emptying. BMC Complement Altern Med. 2019; 19:159. PubMed: https://pubmed.ncbi.nlm.nih.gov/31277639/
- Richter JE, Rubenstein JH. Presentation and Epidemiology of Gastroesophageal Reflux Disease. Gastroenterology. 2018; 154: 267-276. PubMed: https://pubmed.ncbi.nlm.nih.gov/28780072/
- Oustamanolakis P, Tack J. Dyspepsia: organic versus functional. J Clin Gastroenterol. 2012; 46: 175-190. PubMed: https://pubmed.ncbi.nlm.nih.gov/22327302/
- Isobe H, Sakai H, Satoh M, Sakamoto S, Nawata H. Delayed gastric emptying in patients with liver cirrhosis. Dig Dis Sci. 1994; 39: 983-987. PubMed: https://pubmed.ncbi.nlm.nih.gov/8174439/
- Catanzaro R, Calabrese F, Occhipinti S, Anzalone MG, Italia A, et al. Nonalcoholic fatty liver disease increases risk for gastroesophageal reflux symptoms. Dig Dis Sci. 2014; 59: 1939-1945. PubMed: https://pubmed.ncbi.nlm.nih.gov/24718860/
- Llorente C, Jepsen P, Inamine T, Wang L, Bluemel S, et al. Gastric acid suppression promotes alcoholic liver disease by inducing overgrowth of intestinal Enterococcus. Nat Commun. 2017; 16: 837-852. PubMed: https://pubmed.ncbi.nlm.nih.gov/29038503/
- Kalaitzakis E. Gastrointestinal dysfunction in liver cirrhosis. World J Gastroenterol. 2014; 20: 14686–14695. PubMed: https://pubmed.ncbi.nlm.nih.gov/25356031/
- Meng X, Li S, Li Y, Gan RY, Li HB. Gut Microbiota's Relationship with Liver Disease and Role in Hepatoprotection by Dietary Natural Products and Probiotics. Nutrients. 2018; 10: 1-21. PubMed: https://pubmed.ncbi.nlm.nih.gov/30297615/
- Platel K, Srinivasan K. Influence of dietary spices or their active principles on digestive enzymes of small intestinal mucosa in rats. Int J Food Sci Nutr. 1996; 47: 55-59. PubMed: https://pubmed.ncbi.nlm.nih.gov/8616674/
- Loguercio C, Festi D. Silybin and the liver: From basic research to clinical practice. World J Gastroenterol. 2011; 17: 2288–2301. PubMed: https://pubmed.ncbi.nlm.nih.gov/21633595/
- Mala KN, Thomas J, Syam DS, Maliakel B, Krishnakumar IM. Safety and Efficacy of Ferula asafoetida in Functional Dyspepsia: A Randomized, Double-Blinded, Placebo-Controlled Study. Evid Based Complement Alternat Med. 2018: 1-11. PubMed: https://pubmed.ncbi.nlm.nih.gov/30224930/