More Information
Submitted: January 20, 2021 | Approved: January 29, 2021 | Published: February 01, 2021
How to cite this article: Gadour E, Hassan Z. Systematic review and meta-analysis of drug induced liver injury secondary to biologic medications in inflammatory bowel disease. Ann Clin Gastroenterol Hepatol. 2021; 5: 005-012.
DOI: 10.29328/journal.acgh.1001025
ORCiD: orcid.org/0000-0001-5087-1611
Copyright License: © 2021 Gadour E, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Systematic review and meta-analysis of drug induced liver injury secondary to biologic medications in inflammatory bowel disease
Eyad Gadour* and Zeinab Hassan
University Hospitals of Morecambe Bay NHS Foundation Trust, Royal Lancaster Infirmary, England
*Address for Correspondence: Dr. Eyad Gadour, MBBS, MRCP(UK), ESEGH, FRCP(Glasg), University Hospitals of Morecambe Bay NHS Foundation Trust, Royal Lancaster Infirmary, England, Tel: 00447401961196; Email: [email protected]
Introduction: Drug-induced Hepatotoxicity and biologic drugs have historically been challenging in IBD. We aim to study the prevalence of hepatotoxicity in adult patients using biologic medications.
Methodology: With the guidelines described by PRISMA-P, a detailed search strategy for each electronic database was developed based on PubMed, Medline, and Embase. We include RCTs that assessed the efficacy and hepatotoxicity of biologics in IBD patients. Hepatotoxicity was defined as AST and/or ALT >2x upper limit of normal or cholestasis. The Odds ratio (OR) was calculated with a 95% confidence interval (CI). Heterogeneity was assessed using the Chi2 test and the I2 statistic.
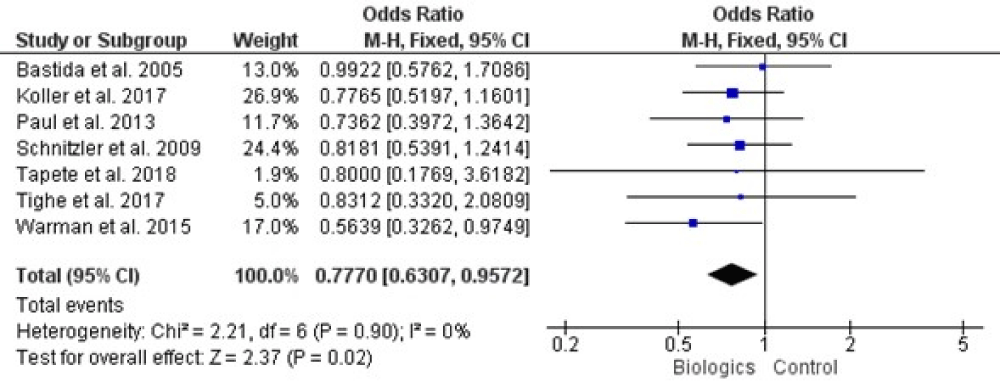
Results: 862 records identified in total. After removing the duplicates 564 records were left for review. Four studies did not report on how participants were randomized to treatment groups or how allocation concealment was achieved, we rated these studies at unclear risk of bias for these domains. There was no presence of any heterogeneity among studies by (Chi2= 2.21, df = 6, p = 0.90, and I2 = 0%). Our meta-analysis was conducted on the fixed effects model, with the (0.770, 95% CI [-0.630, 0.957], and p = 0.02). Hepatotoxicity was not related to any TNF-α antagonist. Thiopurine induced liver injury occurred more frequently within the first months of treatment, 50% of cases within the first 3 months (11.4% vs. 2.3%, p < 0.05).
Conclusion: When hepatotoxicity occurred, the treatment was withdrawn in thirty one percent of patients. This group of patients had a dose-dependent hepatotoxicity rather than an immunologic hepatitis.
Ulcerative colitis (UC) and Crohn’s disease (CD), collectively known as inflammatory bowel disease (IBD), are both characterized by a diffuse inflammation of the bowel [1]. Crohn's disease (CD) is a chronic, episodic, inflammatory condition of the gastrointestinal system, with affected regions consisting of transmural ulceration separated by normal mucosa [2]. The small Intestine is most commonly affected, although the large intestine may also be involved. Common symptoms include abdominal pain, diarrhea, weight loss, bleeding, nausea and vomiting [3]. Abdominal complications may include bowel obstruction, perforation, abscesses, fistulas, and peri-anal disease [4]. Round about twenty percent of people with CD go through extra-intestinal complications that may include musculoskeletal, ocular, dermatologic, hepato-biliary, renal and hematological conditions [5]. On the other hand we have Ulcerative colitis which is an idiopathic inflammatory condition of the colon which results in diffuse friability and superficial erosions on the colonic wall associated with bleeding [6]. It is the most common form of inflammatory bowel disease worldwide [7]. It typically consists of inflammation limited to the mucosa and sub mucosa of the colon [8]. Typically, the disease starts in the rectum and extends proximally in a continuous manner [9]. The cause of inflammatory bowel disease is indistinguishable, but it seems to occur in genetically disposed people in response to environmental factors [10]. Ulcerative colitis is almost certainly an autoimmune disease initiated by an inflammatory response to colonic bacteria [11].Conservative medications for irritable bowel disease such as Ulcerative colitis (UC) and Crohn’s disease (CD), include anti-inflammatory drugs, immune-suppressants and corticosteroids [12]. Still an individual does not respond, or loses response to first-line treatments, then biologic therapies such as tumour necrosis factor-alpha (TNF-G) antagonists such as adalimumab are considered for treating irritable bowel disease [13]. Maintenance of remission of IBD is a clinically important goal, as disease relapse can negatively affect quality of life [14]. Amongst the most commonly prescribed treatments for several chronic inflammatory diseases one of the categories of medications are biologics [15]. Tumor necrosis factor alpha inhibitors, more so than other agents, have been observed to cause drug-induced liver injury. Additionally, because the approval and popularity of checkpoint inhibitors have grown, similar patterns of liver injury have been documented, with a majority of cases describing immune-mediated hepatitis [16]. Although the exact mechanism of injury is unknown, various host and medication characteristics play a role in the outcome of the molecular cascade invoked by biologics [17]. Prognosis is usually favorable with cessation of the damage causing agent, but cases of acute liver failure requiring liver transplantation have also been observed [18]. Therefore, algorithms have been created to assist clinicians in treating drug-induced autoimmune hepatitis, mostly with corticosteroids [19]. Additionally, case reports have documented successfully re-challenging patients with a different biologic without recurrence of liver injury, but data are limited [20]. Further investigation is warranted regarding the potential for cross-reactivity and mechanism of injury to develop guidelines to aid clinicians in further management of these patients [21].
Hepatobiliary disorders are common in patients with inflammatory bowel disease (IBD), and persistent abnormal liver function tests are found in approximately twenty to thirty percent of individuals with IBD. In most cases, the cause of these elevations will fall into 1 of 3 main categories [22].They can be as a result of extraintestinal manifestations of the disease process, related to medication toxicity, or the result of an underlying primary hepatic disorder unrelated to IBD [23]. Biologic therapy to inhibit tumor necrosis factor-alpha (TNF-𝛼), a pro-inflammatory cytokine, has become a widely used, safe, and effective treatment for patients with inflammatory bowel disease (IBD) [24]. For more than the past two decades, biologic therapies have revolutionized the care for people with inflammatory bowel disease (IBD), but each therapy has its own risks, together with the likelihood of liver damage. Numerous classes of biologics for the treatment of IBD now exist [25]. Tumor necrosis factor α (TNF-α) inhibitors were the first biologic class approved for use in 1998. Mechanism of action of these monoclonal antibodies (mAbs) is directed against proinflammatory TNF molecules, which are frequently increased in IBD patients [26]. It has also been reported that he development of anti-drug antibodies with biologic therapy possibly will have positive implications for long-term management [27]. Hence, therefore it was essential to carry out a review study the hepatotoxicity caused by biologics given for treatment of Ulcerative colitis (UC) and Crohn’s disease (CD), in IBD patients.
Search methods for identification of studies
Detailed search strategies for each electronic database were developed based on the one used for Pubmed (Ovid) Medline, and (Ovid) Embase but with appropriate database related search strategy modification such as the use of truncations, wildcards, and filters [28]. The subject search used a combination of the controlled vocabulary terms “Mesh terms” and free-text words based on the search strategy developed for Medline.
We searched the following databases with English language restriction applied in each database until 2020 from the studies inception.
• Medline (Ovid)
• Embase (Ovid)
• PubMed
• Cochrane IBD Group Specialized Register
With the guidelines described by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) [29].
This review covered controlled trials in which biologic was administered to one study group; the control group may or may not have received a placebo.
Criteria for considering studies for this review
Types of studies: We include prospective and retrospective RCTs that assessed the efficacy and hepatotoxicity of biologigcs in IBD patients.
Types of participants: This review includes participants of any age who have been diagnosed with Ulcerative colitis (UC) and Crohn’s disease (CD), using clinical, radiological, endoscopic or histological criteria.
Types of interventions: This review includes trials that compared any biologic either to a placebo or to an active comparator.
Outcome measures: Studies done on efficacy and events of adverse effects, such as liver injury by biologics in IBD patients.
Data collection and analysis
Two review authors independently assessed the titles and abstracts of studies identified by the search criteria to determine eligibility according to the inclusion criteria. We discussed disagreements until we reached a consensus among the review authors, and consulted with a third review author when we could not reach agreement. The characteristics of all included studies are presented in table 1.
| Table 1 | ||||
| Stduy ID | Methods | Participants | Interventions | Outcomes |
| Bastida, et al. 2005 | Prospective randomized study | aged 18 - 75 with Crohn,s disease | 6-mercaptopurine azathioprine | Liver toxicity, Drugs adverse effects |
| Koller, et al. 2017 | Prospective randomized study | Aged 39; 30.0-52.75 with IBD | Infliximab, azathioprine | Mucosal healing, Drugs adverse effects |
| Paul, et al. 2013 | Prospective randomized study | Aged 34.6 6 4.8 with IBD | Infliximab | Drug adverse effects |
| Schnitzler, et al. 2009 | Prospective randomized study | Aged 35.8 (25.7-44.6) with IBD | Infliximab | Drug adverse effects |
| Tapete, et al. 2018 | Prospective randomized study | Aged 35. to 6 4 with Crohn’s Disease | adalimumab (ADL) or infliximab | GI healing, Drug adverse effects |
| Tighe, et al. 2017 | Prospective randomized study | Aged (38-44.) with IBD | adalimumab (ADL) or infliximab | Drug adverse effects |
| Warman, et al. 2015 | Prospective randomized study | Aged (30-53.) with IBD | Infliximab, 6-mercaptopurine azathioprine | Drug adverse effects |
Data extraction and management
Two review authors independently extracted data using a standardized extraction form. We discussed any disagreements over extracted data, and then brought them to a third review author for resolution as required. We extracted the following information:
1. General information (type of publication, title, journal, year).
2. Study design features (method of randomization, concealment of allocation and blinding, power calculation, dates of enrolment and follow-up, study).
3. Eligibility (number of participants screened and theirrandomization).
4. Participant characteristics (age, sex, race, severity of disease,current and prior medications).
5. Intervention (dose and type of medication, and whether it was compared to placebo or active comparator).
6. Primary and secondary outcomes.
7. Follow-up (dates of follow-up along with withdrawals and number of participants lost to follow-up).
8. Funding details and author conflicts of interest.
Assessment of risk of bias in included studies
Two review authors independently assessed risks of bias using the Cochrane 'Risk of bias' tool [30]. We assessed several study characteristics for risks of bias, including random sequence generation, allocation concealment, blinding, incomplete outcome data, and selective outcome reporting and other potential sources of bias. Based on these criteria, we rated the studies as having a low, high or unclear risk of bias for each category. We discussed any disagreements about risks of bias and then brought them to a third review author as necessary.
Statistical analysis
We used Review Manager 5 (RevMan5) to analyze the data. For dichotomous outcomes, we calculated the Odds ratio (OR) with a 95% confidence interval (CI).
Assessment of heterogeneity
We assessed heterogeneity using the Chi2 test and the I2 statistic [31]. We considered an I2 value of less than 25% indicative of low heterogeneity, greater than 50% indicative of moderate heterogeneity and greater than 75% high heterogeneity.
For the Chi2 test, we considered a p value of 0.10 to be statistically significant. If the I2 statistic and Chi2 test suggested heterogeneity, we visually inspected the forest plot for outliers. We used a sensitivity analysis (e.g. excluding outliers) to explore potential explanations for heterogeneity.
Assessment of reporting biases
We used a funnel plot to assess publication bias found between the studies which are presented in figure 3 [32].
Data synthesis
We combined data from individual trials for meta-analysis when interventions, participant groups and outcomes were sufficiently similar. We determined this by discussion and consensus among the review author team. We calculated the pooled OR with a 95% CI for dichotomous outcomes. As there were not a significant heterogeneity between our study we used a fixed-effects model to pool the data.
Sensitivity analysis
We used sensitivity analysis to examine the impact of the following variables on the pooled effect estimate:
1. Random-effects versus fixed-effect modeling.
2. Low risk of bias versus unclear or high risk of bias.
3. Relevant loss to follow-up (more than 10%).
4. Full-text articles versus abstract or unpublished studies.
Description of studies/Results of the search
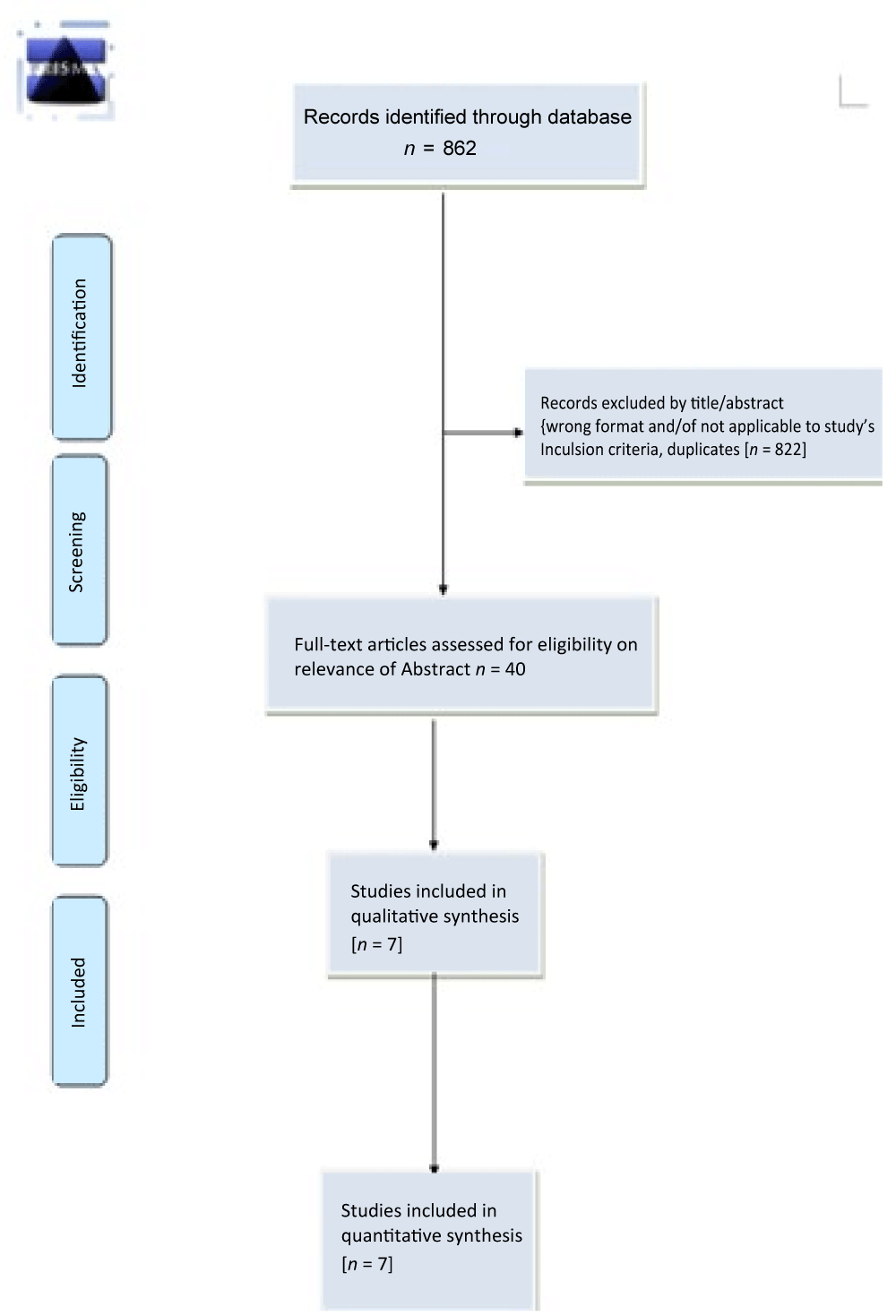
Our literature search identified 862 records in total. After we had removed duplicates 564 records were left for review. Two review authors (MK and SK) independently reviewed the titles and abstracts of these records and selected 40 full-text articles for review, we further excluded thirty one studies with different reasons and finally seven studies [33-39] (total of 896 participants) met the predefined inclusion criteria and were included in this review (Figure 1).
Figure 1: Prisma Flow Diagram.
Risk of bias in included studies
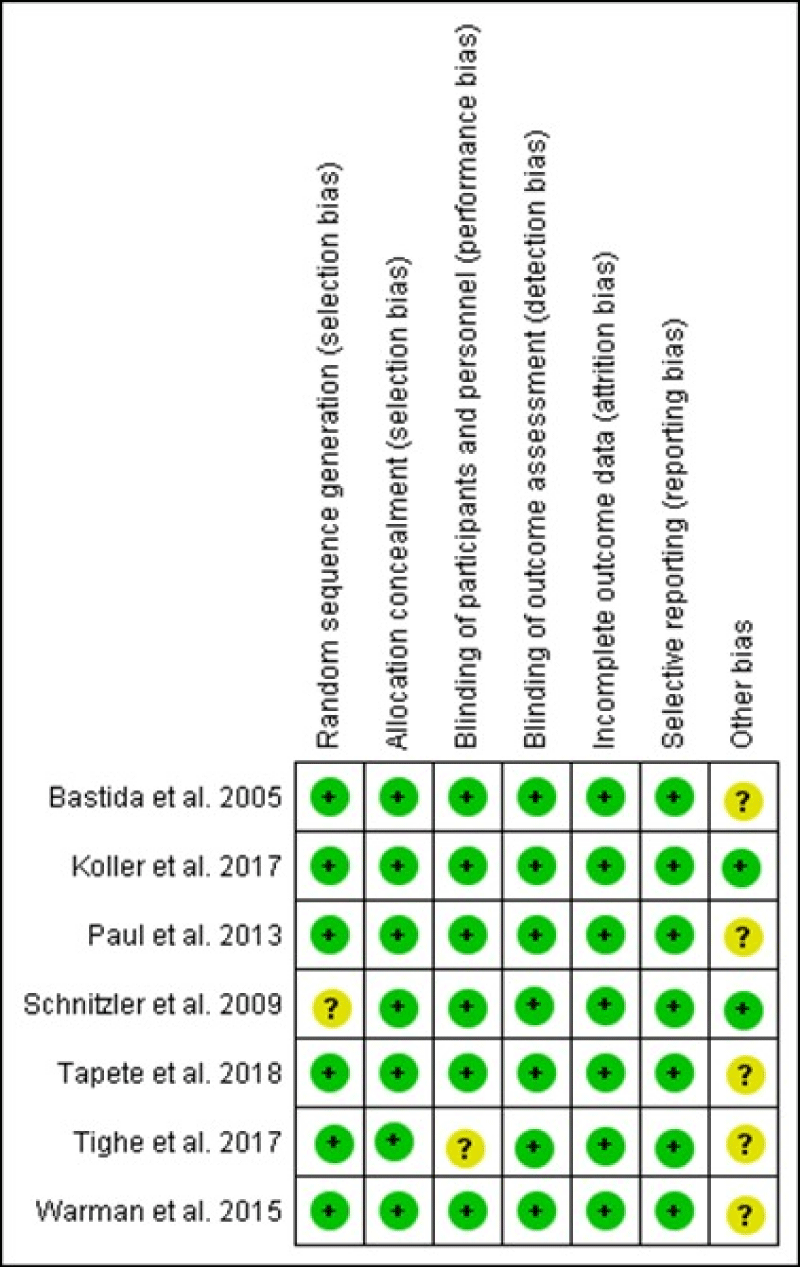
We assessed the methodological quality of each study using the Cochrane 'Risk of bias' tool and we summarize our findings in figure 2.
Figure 2: Risk of bias summary.
Allocation
Four studies did not report on how participants were randomized to treatment groups or how allocation concealment was achieved. We rated these studies at unclear risk of bias for these domains [35,36,38,39].
Blinding
Two studies did not report on how blinding was maintained for participants, personnel or outcome assessors throughout the study time period. We rated these studies at unclear risk of bias for these domains [38,40].
Incomplete outcome data
One study [39] did not report on the number of participants who were initially randomized, so we could not determine the number of participants who had withdrawn during the study period. We rated the study at unclear risk of bias for this domain.
Selective reporting
All of the included studies reported on all expected outcomes and were rated at low risk of bias for this domain.
Other potential sources of bias
Five of the included studies appeared to have other potential sources of bias and were rated at low risk of bias for this domain [35,36,38,39].
Result of pooled data
All of our included trials mentioned adverse effect of biologics on liver which are analyzed statistically and result is summarized in figure 3, there was not presence of any heterogeneity among studies by (Chi2= 2.21, df = 6, p = 0.90, and I2= 0%), when the whole seven studies were involved for analysis. Our meta-analysis was conducted on the fixed effects model, with the (0.770, 95% CI [-0.630, 0.957], and p = 0.02).
Figure 3: Forest plot of all included studies.
Publication bias
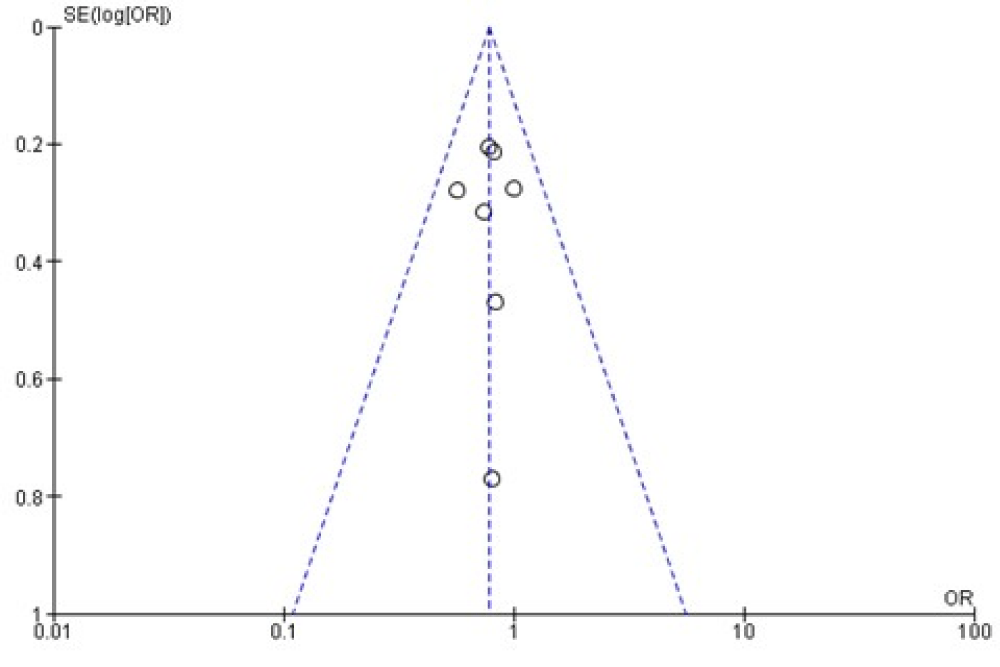
The funnel plot was generated based on the mean adverse events; funnel plot was applied to evaluate the publication biases of all seven studies. Summarized in figure 4, the outcome suggests that there was not significant publication bias.
Figure 4: Funnel plot.
This review includes seven studies which are prospective observational studies that examined the efficacy and safety of biologics in IBD patients. Warman, et al. 2015 discussed therapeutic drug monitoring of infliximab is not common care in the daily practice of a gastroenterologist treating IBD. Lack of effectiveness or the manifestation of side effects may often be encountered by dose or interval adjustments before turning to therapeutic drug monitoring. Therefore, these trough levels are not based on a standard regime, as we see in patients newly started on infliximab, but might be influenced by the adjustments [41]. Their study provided insights into infliximab trough levels in our IBD cohort in which dose adjustments had already been performed and whether there is still an association with remission [39]. In 2005 Bastida, et al. mentioned first time about incidence of hepatotoxicity in IBD patients treated with thiopurinic immunomodulators [42]. The incidence of abnormal LFTs or liver toxicity is a relevant finding during the follow-up of patients treated with thiopurinic immune-modulators as it was showed in their study conducted with a cohort of 161 IBD patients [43]. There is a lack of recent published series that specifically assess thiopurine-induced hepatotoxicity, but some recent studies with IBD patients do not describe any case of liver injury during the follow-up [33]. Probably the main limitation of studies evaluating drug-induced liver toxicity is related with diagnosis, due to the absence of specific markers or tests. Therefore, the diagnosis relied entirely on circumstantial evidence and only in the cases of relapse after rechallenge we had the certainty that azathioprine or mercaptopurine were the offending drug [44].Liver biopsy is an invasive procedure with significant morbidity and was not performed routinely to all patient presented with abnormal LFTs. However, liver biopsy is not required to establish the diagnosis of drug-induced liver toxicity. Based on the absence of histological confirmation and in the fact that an important percentage of patients were able to tolerate full-dose therapy, we cannot assert that we are handling with a true hepatotoxicity which implies hepatocyte damage rather than a form of tolerance. It is important to remark that we ruled out other causes that might have explained the liver injury as well as alcohol or hepatotoxic drugs intake, but we should be aware that the patient could be hiding the consumption of illegal drugs or herbal remedies [45]. In their study Paul, et al. reported that ATI levels were associated with loss of response to infliximab. The ELISA used in their study was able to assess ATI levels independently from IFX trough concentrations. This may partly explain the discrepancy between our results and previous reports [46,47]. Immuno-monitoring has been increasingly recognized as a useful tool to explore an immune basis behind LOR to anti- TNFa therapy. It can be used alongside other biochemical predictors of LOR such as CRP and faecal calprotectin [48]. In their one retrospective study Tighe, et al. 2017 analyzed patients who previously had stand-alone anti-TNFa trough and antibodies measured [38]. They aimed to see whether these stand-alone anti-TNFa trough and antibody levels would be useful in predicting future outcomes [38]. Similar to other studies, a significant number of their cohort treated with anti-TNFa had a negative outcome (twenty-seven percent 20/74) [14,49], it is important to remark that we ruled out other causes that might have explained the liver injury as well as alcohol or hepatotoxic drugs intake, but we should be aware that the patient could be hiding the consumption of illegal drugs or herbal remedies [50]. It is worth noting some considerations related to the clinical course of thiopurine-induced liver injury. At first it is important to point out that a small percentage of patients, less than five percent presented with a slight elevation of LFTs that did not have clinical implications: the abnormalities in liver chemical tests returned to normal values during the follow-up and was not necessary to adjust dose of immunomodulator [51]. As with other drugs, thiopurine induced liver injury occurred more frequently within the first months of treatment, 50% of cases within the first 3 months [52]. Moreover, treatment withdrawal because of hepatotoxicity occurred in most of cases, 75%, during this period of time [53]. Despite this, in some cases the liver injury was only detected after a long period of follow-up leading to therapy withdrawal [54]. This finding is surprising because the long delay makes the role of the suspected drug unlikely; the explanation of this event could be related to a cumulative effect of the metabolites on liver or to the confluence of multiple factors that could be triggers of an autoimmune liver injury. We found acute hepatocellular hepatitis in eighty seven percent of patients, in contrast with previous descriptions that considered pure cholestasis as the typical pattern [55]. In all cases LFTs returned to normal values and no chronic disease was detected [56]. When hepatotoxicity occurred, the treatment was withdrawn in thirty one percent of patients, but an important percentage, forty four was able to continue on full dose of thiopurine once the dose was temporarily adjusted [57]. This group of patients had a dose-dependent hepatotoxicity rather than an immune-allergic hepatitis [58]. The rationale about how these patients were able to return to full doses of thiopurinic immune-modulators may be theoretically explained by the confluence of multiple factors in the onset of hepatotoxicity: dose of immunomodulator, concomitant treatment, quality of nutrition, drug interaction, etc.. [59]. Schnitzler, et al. 2009 postulated that the variation in injury pattern could be secondary to variables such as concomitant medications or dosage of medications [60]. Dosage of TNF-𝛼 antagonists did not correlate with liver injury in our case series [61]. Amongst our patients, Subject 1 received therapy with high dose infliximab (10 mg/kg every 8 weeks) when hepatotoxicity was documented. However, Subjects 2 and 3 received standard doses of infliximab (5 mg/kg every 8 weeks) and standard induction dosing of adalimumab (160 mg at week 0, 80 mg at week 2, and 40 mg every other week thereafter), respectively, when liver injury was noticed. Latency time to the development of liver toxicity was also variable. Some cases, liver toxicity developed after eighteen months of infliximab, whereas toxicity developed within three months in some cases [62]. Hepatotoxicity was not related to any particular TNF-𝛼 antagonist, and patient age also varied among our three patients [63]. The variability of histology, dosage, time to toxicity, and presence of concomitant medications, in our patients as well as in review of published cases, highlights the idiosyncratic nature of this drug-induced liver injury [1]. As clinician awareness of this entity increases, and more cases are detected, hopefully distinct patterns of injury will be delineated so that early detection can take place and fulminant liver failure can be prevented [64].
Quality of the evidence
Two of the included studies were judged to be at low risk of bias [38,40]. Four studies were rated at unclear risk of bias for random sequence generation and allocation concealment [33,35,38,60]. Another two studies were found at unclear risk of bias for blinding [33,36] and one study at unclear risk of bias for incomplete outcome data [39].
Potential biases in the review process
A comprehensive literature review to help ensure that we included all relevant studies. Two review authors independently assessed for study inclusion, extracted data and assessed for risks of bias. The main limitation of this review is the lack of data available for endoscopic and histological end points.
This study summed up the broad information of incidences of biologic related hepatotoxicity in IBD patients in clinical practice setting. When hepatotoxicity occurred, the treatment was withdrawn in thirty one percent of patients, but an important percentage, forty-four was able to continue full dose of thiopurine once the dose was temporarily adjusted. This group of patients had a dose-dependent hepatotoxicity rather than an immunologic hepatitis. Further studies are required to look into dose related hepatotoxicity in different stages of inflammatory bowel diseases especially patients who underwent surgical interventions.
- Feagan B, Sandborn WJ, Rutgeerts P, Levesque BG, Khanna R, et al. Performance of Crohn's disease Clinical Trial Endpoints based upon Different Cutoffs for Patient Reported Outcomes or Endoscopic Activity: Analysis of EXTEND Data. Inflamm Bowel Dis. 2018; 24: 932-942. PubMed: https://pubmed.ncbi.nlm.nih.gov/29668919/
- Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011; 141: 1194-1201. PubMed: https://pubmed.ncbi.nlm.nih.gov/21723220/
- Szamosi T, Banai J, Lakatos L, Czegledi Z, David G, et al. Early azathioprine/biological therapy is associated with decreased risk for first surgery and delays time to surgery but not reoperation in both smokers and nonsmokers with Crohn's disease, while smoking decreases the risk of colectomy in ulcerative colitis. Eur J Gastroenterol Hepatol. 2010; 22: 872-879. PubMed: https://pubmed.ncbi.nlm.nih.gov/19648821/
- Wilhelm SM, McKenney KA, Rivait KN, Kale-Pradhan PB. A review of infliximab use in ulcerative colitis. Clin Ther. 2008; 30: 223-230. PubMed: https://pubmed.ncbi.nlm.nih.gov/18343261/
- Vatn MH. Mucosal healing: impact on the natural course or therapeutic strategies. Dig Dis. 2009; 27: 470-475. PubMed: https://pubmed.ncbi.nlm.nih.gov/19897962/
- Rutgeerts P, Diamond RH, Bala M, Olson A, Lichtenstein GR, et al. Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn's disease. Gastrointest Endosc. 2006; 63: 433-442. PubMed: https://pubmed.ncbi.nlm.nih.gov/16500392/
- Burisch J. Crohn's disease and ulcerative colitis. Occurrence, course and prognosis during the first year of disease in a European population-based inception cohort. Dan Med J. 2014; 61: B4778. PubMed: https://pubmed.ncbi.nlm.nih.gov/24393595/
- Fratila OC, Craciun C. Ultrastructural evidence of mucosal healing after infliximab in patients with ulcerative colitis. J Gastrointestin Liver Dis. 2010; 19: 147-153. PubMed: https://pubmed.ncbi.nlm.nih.gov/20593047/
- Romberg-Camps MJ, Dagnelie PC, Kester AD, Hesselink-van de Kruijs MAM, Cilissen M, et al. Influence of phenotype at diagnosis and of other potential prognostic factors on the course of inflammatory bowel disease. Am J Gastroenterol. 2009; 104: 371-383. PubMed: https://pubmed.ncbi.nlm.nih.gov/19174787/
- Henriksen M, Jahnsen J, Lygren I, Aadland E, Schulz T, et al. Clinical course in Crohn's disease: results of a five-year population-based follow-up study (the IBSEN study). Scand J Gastroenterol. 2007; 42: 602-610. PubMed: https://pubmed.ncbi.nlm.nih.gov/17454881/
- Van Assche G. Does mucosal healing impact patient outcomes long-term? Inflamm Bowel Dis. 2008; 14: 577-578. PubMed: https://pubmed.ncbi.nlm.nih.gov/18240284/
- Khanna R, Zou G, D'Haens G, Sandborn WJ, Vandervoort MK, et al. A retrospective analysis: the development of patient reported outcome measures for the assessment of Crohn's disease activity. Aliment Pharmacol Ther. 2015; 41: 77-86. PubMed: https://pubmed.ncbi.nlm.nih.gov/25348809/
- Seow CH, Newman A, Irwin SP, Steinhart AH, Silverberg MS, Greenberg GR. Trough serum infliximab: a predictive factor of clinical outcome for infliximab treatment in acute ulcerative colitis. Gut. 2010; 59: 49-54. PubMed: https://pubmed.ncbi.nlm.nih.gov/19651627/
- Scheinfeld N. Adalimumab: a review of side effects. Expert Opin Drug Saf. 2005; 4: 637-641. PubMed: https://pubmed.ncbi.nlm.nih.gov/16011443/
- Vande Casteele N, Ferrante M, Van Assche G, Ballet V, Compernolle G, et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015; 148: 1320-1329. PubMed: https://pubmed.ncbi.nlm.nih.gov/25724455/
- Ben-Horin S. Drug Level-based Anti-Tumor Necrosis Factor Therapy: Ready for Prime Time? Gastroenterology. 2015; 148: 1268-1271. PubMed: https://pubmed.ncbi.nlm.nih.gov/25921372/
- Chanson P, Pariente A. [Adapting the dose to the residual concentration of infliximab in cryptogenic inflammatory diseases of the intestines]. Rev Prat. 2015; 65: 473.
- Orfanoudaki E, Gazouli M, Foteinogiannopoulou K, et al. Infliximab trough levels are decreasing over time in patients with inflammatory bowel disease on maintenance treatment with infliximab. Eur J Gastroenterol Hepatol. 2019; 31: 187-191. PubMed: https://pubmed.ncbi.nlm.nih.gov/30543573/
- Beppu T, Ono Y, Matsui T, Hirai F, Yano Y, et al. Mucosal healing of ileal lesions is associated with long-term clinical remission after infliximab maintenance treatment in patients with Crohn's disease. Dig Endosc. 2015; 27: 73-81. PubMed: https://pubmed.ncbi.nlm.nih.gov/24833527/
- Shah P, Sundaram V, Björnsson E. Biologic and Checkpoint Inhibitor‐Induced Liver Injury: A Systematic Literature Review. Hepatol Commun. 2020; 4: 172–184. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6996412/
- Peyrin-Biroulet L, Ferrante M, Magro F, Campbell S, Franchimont D, et al. Results from the 2nd Scientific Workshop of the ECCO. I: Impact of mucosal healing on the course of inflammatory bowel disease. J Crohns Colitis. 2011; 5: 477-483. PubMed: https://pubmed.ncbi.nlm.nih.gov/21939925/
- Navaneethan U, Shen B. Hepatopancreatobiliary manifestations and complications associated with inflammatory bowel disease. Inflamm Bowel Dis. 2010; 16: 1598-1619. PubMed: https://pubmed.ncbi.nlm.nih.gov/20198712/
- Raj V, Lichtenstein DR. Hepatobiliary manifestations of inflammatory bowel disease. Gastroenterol Clin North Am. 1999; 28: 491-513. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10372279
- Rojas-Feria M, Castro M, Suárez E, Ampuero J, Romero-Gómez M. Hepatobiliary manifestations in inflammatory bowel disease: the gut, the drugs and the liver. World J Gastroenterol. 2013; 19: 7327-7340. PubMed: https://pubmed.ncbi.nlm.nih.gov/24259964/
- Lichtenstein DR. Hepatobiliary complications of inflammatory bowel disease. Curr Gastroenterol Rep. 2011; 13: 495-505. PubMed: https://pubmed.ncbi.nlm.nih.gov/21773706/
- Jiang Y, Lin O, Sinha SR. Use of Tumor Necrosis Factor Alpha Inhibitors for Inflammatory Bowel Disease Patients with Concurrent Heart Failure. Dig Dis Sci. 2017; 62: 1597-1606. PubMed: https://pubmed.ncbi.nlm.nih.gov/28417241/
- Magro F, Portela F. Management of inflammatory bowel disease with infliximab and other anti-tumor necrosis factor alpha therapies. BioDrugs. 2010; 24 Suppl1: 3-14. PubMed: https://pubmed.ncbi.nlm.nih.gov/21175228/
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 6: e1000097. PubMed: https://pubmed.ncbi.nlm.nih.gov/19621072/
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med. 2009; 3: e123-130.
- Higgins JP AD, Sterne JA. Chapter 8: Assessing, risk of bias in included studies. In: Higgins JP CR, Chandler J CM, editor(s). Cochrane Handbook for, (updated SRoIv, June 2017). The Cochrane Collaboration Af. www.training.cochrane.org/handbook.
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343: d5928. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3196245/
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315: 629-634. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2127453/
- Bastida G, Nos P, Aguas M, Beltrán B, Rubín A, et al. Incidence, risk factors and clinical course of thiopurine-induced liver injury in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2005; 22: 775-782. PubMed: https://pubmed.ncbi.nlm.nih.gov/16225485/
- Koller T, Galambosova M, Filakovska S, Kubincova M, Hlavaty T, et al. Drug-induced liver injury in inflammatory bowel disease: 1-year prospective observational study. World J Gastroenterol. 2017; 23: 4102–4111. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5473129/
- Paul S, Del Tedesco E, Marotte H, Rinaudo-Gaujous M, Moreau A, et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm Bowel Dis. 2013; 19: 2568-2576. PubMed: https://pubmed.ncbi.nlm.nih.gov/24013361/
- Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn's disease. Inflamm Bowel Dis. 2009; 15: 1295-1301. PubMed: https://pubmed.ncbi.nlm.nih.gov/19340881/
- Bertani L, Bodini G, Mondello G, Mumolo MG. Crohn’s disease patients treated with anti-TNF: a prospective multi-centre study. 2019; 13(Supplement_1):S402-S403.
- Tighe D, Hall B, Jeyarajah SK, Smith S, Breslin N, et al. One-Year Clinical Outcomes in an IBD Cohort Who Have Previously Had Anti-TNFa Trough and Antibody Levels Assessed. Inflamm Bowel Dis. 2017; 23: 1154-1159. PubMed: https://pubmed.ncbi.nlm.nih.gov/28486256/
- Warman A, Straathof JW, Derijks LJ. Therapeutic drug monitoring of infliximab in inflammatory bowel disease patients in a teaching hospital setting: results of a prospective cohort study. Eur J Gastroenterol Hepatol. 2015; 27: 242-248. PubMed: https://pubmed.ncbi.nlm.nih.gov/25569569/
- Koller T, Galambosova M, Filakovska S, Kubincova M, Hlavaty T, et al. Drug-induced liver injury in inflammatory bowel disease: 1-year prospective observational study. World J Gastroenterol. 2017; 23: 4102-4111. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5473129/
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005; 353: 2462-2476. PubMed: https://pubmed.ncbi.nlm.nih.gov/16339095/
- Goldstein ES, Marion JF, Present DH. 6-Mercaptopurine is effective in Crohn's disease without concomitant steroids. Inflamm Bowel Dis. 2004; 10: 79-84. PubMed: https://pubmed.ncbi.nlm.nih.gov/15168805/
- de Jong DJ, Goullet M, Naber TH. Side effects of azathioprine in patients with Crohn's disease. Eur J Gastroenterol Hepatol. 2004; 16: 207-212. PubMed: https://pubmed.ncbi.nlm.nih.gov/15075996/
- Larrey D. Epidemiology and individual susceptibility to adverse drug reactions affecting the liver. Semin Liver Dis. 2002; 22: 145-155. PubMed: https://pubmed.ncbi.nlm.nih.gov/12016546/
- Larrey D. Drug-induced liver diseases. J Hepatol. 2000; 32(1 Suppl): 77-88. PubMed: https://pubmed.ncbi.nlm.nih.gov/10728796/
- Maser EA, Villela R, Silverberg MS, Greenberg GR. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn's disease. Clin Gastroenterol Hepatol. 2006; 4: 1248-1254. PubMed: https://pubmed.ncbi.nlm.nih.gov/16931170/
- Rutgeerts P, Vermeire S, Van Assche G. Predicting the response to infliximab from trough serum levels. Gut. 2010; 59: 7-8. PubMed: https://pubmed.ncbi.nlm.nih.gov/20007955/
- Ben-Horin S, Yavzori M, Katz L, et al. The immunogenic part of infliximab is the F(ab')2, but measuring antibodies to the intact infliximab molecule is more clinically useful. Gut. 2011; 60: 41-48. PubMed: https://pubmed.ncbi.nlm.nih.gov/20519742/
- Boschetti G, Garnero P, Moussata D, et al. Accuracies of serum and fecal S100 proteins (calprotectin and calgranulin C) to predict the response to TNF antagonists in patients with Crohn's disease. Inflamm Bowel Dis. 2015; 21: 331-336. PubMed: https://pubmed.ncbi.nlm.nih.gov/25625487/
- Menor C, Fernández-Moreno MD, Fueyo JA, et al. Azathioprine acts upon rat hepatocyte mitochondria and stress-activated protein kinases leading to necrosis: protective role of N-acetyl-L-cysteine. J Pharmacol Exp Ther. 2004; 311: 668-676. PubMed: https://pubmed.ncbi.nlm.nih.gov/15226385/
- Tapner MJ, Jones BE, Wu WM, Farrell GC. Toxicity of low dose azathioprine and 6-mercaptopurine in rat hepatocytes. Roles of xanthine oxidase and mitochondrial injury. J Hepatol. 2004; 40: 454-463. PubMed: https://pubmed.ncbi.nlm.nih.gov/15123360/
- Choudhury J, Sanyal AJ. Insulin resistance and the pathogenesis of nonalcoholic fatty liver disease. Clin Liver Dis. 2004; 8: 575-594. PubMed: https://pubmed.ncbi.nlm.nih.gov/15331065/
- Farrell GC. Drugs and steatohepatitis. Semin Liver Dis. 2002; 22: 185-194. PubMed: https://pubmed.ncbi.nlm.nih.gov/12016549/
- Morris JM, Oien KA, McMahon M, Forrest EH, Morris J, et al. Nodular regenerative hyperplasia of the liver: survival and associated features in a UK case series. Eur J Gastroenterol Hepatol. 2010; 22: 1001-1005. PubMed: https://pubmed.ncbi.nlm.nih.gov/20075739/
- Bénichou C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990; 11: 272-276. PubMed: https://pubmed.ncbi.nlm.nih.gov/2254635/
- Lennard-Jones JE. Classification of inflammatory bowel disease. Scand J Gastroenterol Suppl. 1989; 170: 2-6. PubMed: https://pubmed.ncbi.nlm.nih.gov/2617184/
- Weersma RK, Peters FT, Oostenbrug LE, van den Berg AP, van Haastert M, et al. Increased incidence of azathioprine-induced pancreatitis in Crohn's disease compared with other diseases. Aliment Pharmacol Ther. 2004; 20: 843-850. PubMed: https://pubmed.ncbi.nlm.nih.gov/15479355/
- Marion JF. Toxicity of 6-mercaptopurine/azathioprine in patients with inflammatory bowel disease. Inflamm Bowel Dis. 1998; 4: 116-117. PubMed: https://pubmed.ncbi.nlm.nih.gov/9687219/
- Present DH, Meltzer SJ, Krumholz MP, Wolke A, Korelitz BI. 6-Mercaptopurine in the management of inflammatory bowel disease: short- and long-term toxicity. Ann Intern Med. 1989; 111: 641-649. PubMed: https://pubmed.ncbi.nlm.nih.gov/2802419/
- Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn's disease: results from a single-centre cohort. Gut. 2009; 58: 492-500. PubMed: https://pubmed.ncbi.nlm.nih.gov/18832518/
- Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010; 362: 1383-1395. PubMed: https://pubmed.ncbi.nlm.nih.gov/20393175/
- Roblin X, Marotte H, Leclerc M, Del Tedesco E, Phelip JM, et al. Combination of C-reactive protein, infliximab trough levels, and stable but not transient antibodies to infliximab are associated with loss of response to infliximab in inflammatory bowel disease. J Crohns Colitis. 2015; 9: 525-531. PubMed: https://pubmed.ncbi.nlm.nih.gov/25895875/
- Imaeda H, Bamba S, Takahashi K, Fujimoto T, Ban H, et al. Relationship between serum infliximab trough levels and endoscopic activities in patients with Crohn's disease under scheduled maintenance treatment. J Gastroenterol. 2014; 49: 674-682. PubMed: https://pubmed.ncbi.nlm.nih.gov/23666424/
- Frøslie KF, Jahnsen J, Moum BA, Vatn MH. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007; 133: 412-422. PubMed: https://pubmed.ncbi.nlm.nih.gov/17681162/