More Information
Submitted: December 17, 2020 | Approved: January 08, 2021 | Published: January 11, 2021
How to cite this article: Issa RR, Sira AM, Sira MM. Stem cell therapy in children with acute liver failure: The dream could come true. Ann Clin Gastroenterol Hepatol. 2021; 5: 001-004.
DOI: 10.29328/journal.acgh.1001024
Copyright License: © 2021 Issa RR, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Stem cell therapy in children with acute liver failure: The dream could come true
Riham Rabie Issa*, Ahmad Mohamed Sira and Mostafa Mohamed Sira
Department of Pediatric Hepatology, Gastroenterology, and Nutrition1, National Liver Institute, Menoufia University, 32511 Shebin El-Koom, Menoufia, Egypt
*Address for Correspondence: Riham Issa, Department of Pediatric Hepatology, Gastroenterology, and Nutrition, National Liver Institute, Menoufia University, 32511 Shebin El-Koom, Menoufia, Egypt, Fax: +2-048-223-4586; Tel: +2-048-222-2740; Email: [email protected]
Acute liver failure (ALF) in children is a severe disease with a high mortality rate. The current treatment strategies are still defective, with many cases die when liver transplantation is unavailable. The current protocol of steroids therapy improved the survival rate of hepatitis A virus (HAV)-related ALF. However, there is still a high mortality for non-HAV cases. Stem cell therapy (SCT) has been tried in experimental animals with ALF and in few adult studies with acute-on-chronic liver failure. No previous trials of SCT have been tested in children with ALF. The absence of SCT application in ALF in children could be due to some issues. These could be related to safety, sources, administration route, optimum dosage, efficacy, and survival. It is proposed that could be the future therapy if these obstacles have been well studied and solved.
Despite the improvement of acute liver failure (ALF) survival in children with fulminant hepatitis A (from 27% to 83%) after our recent protocol of steroids in its treatment [1], there is still a high mortality in non-hepatitis A virus (HAV)-related ALF. When liver transplantation (LT) is not feasible, the standard medical therapy (SMT) and other supportive measures as liver dialysis are insufficient to achieve recovery. Even when LT is possible, it carries many significant risks [2]. Within the last eight months, the mortality of non-HAV patients with ALF in our center reached 60%.
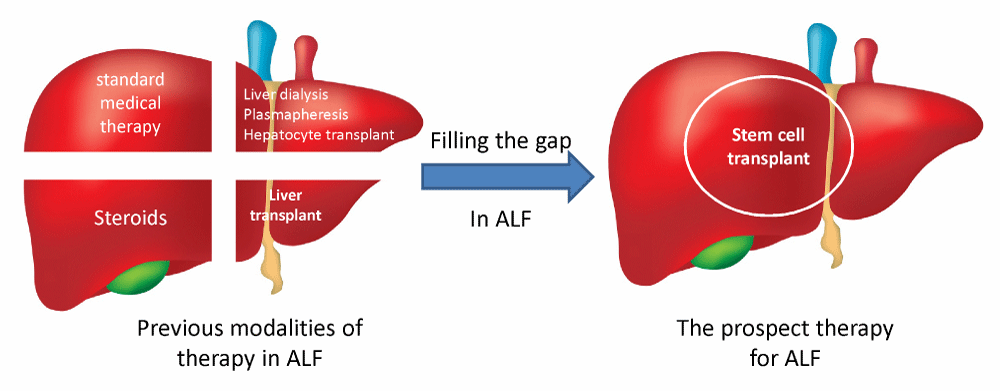
Due to ALF’s insufficient therapeutic modalities, a new therapeutic line needs to be introduced to fill the gap between those responding to SMT plus steroids and those waiting for LT with other supportive measures as liver dialysis (Figure 1).
Figure 1: Illustration of previous and future modalities of acute liver failure therapy in children.
It has been shown that the stem cell (SC) has a hopeful therapeutic effect in liver disease [3]. However, no previous trials of SC therapy (SCT) have been accomplished in children with ALF. It is a dream that SCT could be the solution to this critical situation.
SCT has been used in many pediatric diseases. It achieved a dramatic improvement in pediatric hematological oncology in the past few years. The American and European societies for blood and marrow transplantation listed many indications for SCT in children, including hematological malignancies (e.g., acute lymphocytic leukemia, acute myeloid leukemia, and Hodgkin lymphoma) and non-malignant disorders, including hemoglobinopathies (e.g., thalassemia and sickle cell disease). Also, severe combined immunodeficiency, chronic granulomatous disease, Fanconi anemia, and severe aplastic anemia are among the reported indications [4,5].
Moreover, SCT has been used in the lysosomal storage diseases as Hurler’s syndrome [6]. Also, it was reported in Ewing’s sarcoma, Wilms tumor, and neuroblastoma [7].
The lack of SCT clinical application in ALF in children could be due to many obstacles; if solved, it could be the future of this fatal disease.
Stem cells (SCs) are undifferentiated cells with self-renewal ability. They can differentiate into specialized cells and also have anti-apoptotic and anti-fibrotic activity. It plays a significant role in regenerative medicine nowadays, despite many concerns like carcinogenesis and low survival that requires extensive research [8].
Many SCs’ sources are available, namely, embryonic, which has a significant ethical issue, fetal, umbilical cord (UC), placental, and adult stem cells. The most commonly isolated adult SCs are those obtained from peripheral blood, bone marrow (BM), and adipose tissue [9].
SCT has been proposed as a therapeutic alternative for ALF. However, all studies were preclinical ones that have been applied in animals. These studies proved SCT’s efficacy in improving ALF in animals by different mechanisms; the hepatocyte-like cell transformations and the immunomodulation action. Despite the number of these preclinical studies on animal models of ALF [10-12] and the few clinical ones in adults with acute-on-chronic liver failure (ACLF) [13,14], there are still many unsolved issues. These are the safety, efficacy, route of administration, immunosuppression, and oncogenicity. Other problems are the need for a bridge until SCs’ therapy effectiveness and which type and source of SCs are preferable; autologous or allogenic.
In one of the few clinical studies, Lin, et al. [15] tried allogeneic BM-derived mesenchymal stromal cells (MSCs) to treat 56 adults with hepatitis B virus-related ACLF. They infused 1.0 to 10 × 105 cells/kg allogeneic BM-derived MSCs weekly for four weeks and then followed for 24 weeks. The survival rate was 73% vs. 55% in the control group (p = 0.03). Compared with the control group, allogeneic BM-derived MSC treatment markedly improved clinical and laboratory measurements, including serum total bilirubin and Model for End-Stage Liver Disease (MELD) scores. The incidence of severe infection in the MSC group was much lower than that in the control group (16.1% vs. 33.3%, p = 0.04). Mortality from multiple organ failure and severe infection was higher in the control group than in the MSC group (37.0% vs. 17.9%, p = 0.02).
All SC research goals are to find the best source and to test efficacy and safety in humans in different diseases [8]. MSCs, one of the adult SCs, can differentiate into hepatocyte-like cells and have immunomodulation capacity through secretions of many factors. Thus, MSCs participate in liver repair through direct and indirect mechanisms [3].
Stem cells sources
It is a dilemma of which source is better to use; UC, BM, or peripheral blood. Some researchers consider that BM and UC are the best two SC sources until now in humans [9]. BM transplantation (BMT) had been used a lot before in SCT, but according to liver conditions, Zhong, et al. [16] showed impaired proliferation of BM-derived MSCs in cirrhotic liver and chronic hepatitis B.
UC blood SCs are described to have some advantages over that of BM. For example, it can be easily collected with no hazards on donor, availability, low rate of rejection, greater proliferation capacity, minimal risk of viral transmission, and using a previously prepared UC-derived SCs may be beneficial not to lose time for processing in rapidly deteriorating cases [17].
On the other hand, peripheral blood-derived SCs need some time for their separation. The patient or a sibling is given stimulating factors to get many SCs in the blood [18]. This couldn’t save a rapidly deteriorating patient with limited available time to start SCT. Lastly, the embryonic and fetal SCs have significant ethical issues [19].
Route of injection
Different routes for injection of SCs are described in hepatic patients, like peripheral intravenous (IV), intrahepatic, intraportal, and intrasplenic. The best injection route is not standardized yet. Different administration routes were used in clinical trials. It seems that the IV route is the easiest and safest without the need for an expert on invasive techniques. A comparison of routes to choose which is the best is difficult as each trial has its dosage and different source [9]. Efficacy was reported to be slightly affected according to the administration’s route [3].
Amer, et al. [20] compared intrahepatic with intrasplenic BM-derived SCs administration route in end-stage liver failure patients with no statistically significant difference between both. However, the splenic route was slightly easier but with a higher incidence of mild complications. El-Ansary, et al. [21] also compared the splenic versus the IV route for BM-derived SCs administration in end-stage liver failure with no significant difference in results.
Meanwhile, it seems that the IV and invasive techniques like injection of SCs through splenic, portal, or hepatic artery are equal to each other [9].
Dose and repetition
SCs dosage was given around 1-2 million cells/kg in most clinical conditions and didn’t exceed 12 million cells/kg [22]. Others suggested that the dosage should be adjusted according to the severity, and even it may be repeated and supposed to have a better outcome with earlier repetition.
Safety
Most of the studies are short term studies, with few long term ones up to 66 months. Most of the short-term studies showed safety. Some long term studies showed complications as hepatocellular carcinoma (HCC) development after autologous BMT in 5 patients out of 19 (26.3%); one case had it before SCT and removed surgically, the other four cases developed at 16, 17, 18, and 56 months after SCT [23]. However, more studies are still needed to detect long-term complications, especially in children with long life expectancy.
Efficacy
Most of the studies showed efficacy in many liver diseases like chronic liver failure, ALF post hepatitis B, and graft rejection post-transplantation. Outcomes were assessed according to different study measures, with the most important like bilirubin, prothrombin time, albumin, MELD score, ascites, lower limb edema, and encephalopathy [3].
Improving SCs effectiveness
Kang, et al. [24] illustrated some ways to improve SCT’s effectiveness that can be followed, like tissue engineering, preconditioning to improve cell resistance, and genetic engineering.
Survival
Some studies showed survival for up to one year [25]. However, in pediatric ALF, it is supposed that the need for SCs would be temporary to overcome the massive acute destruction of hepatocytes. So it is expected that the result will be better than in chronic liver diseases.
Proposed treatment strategy
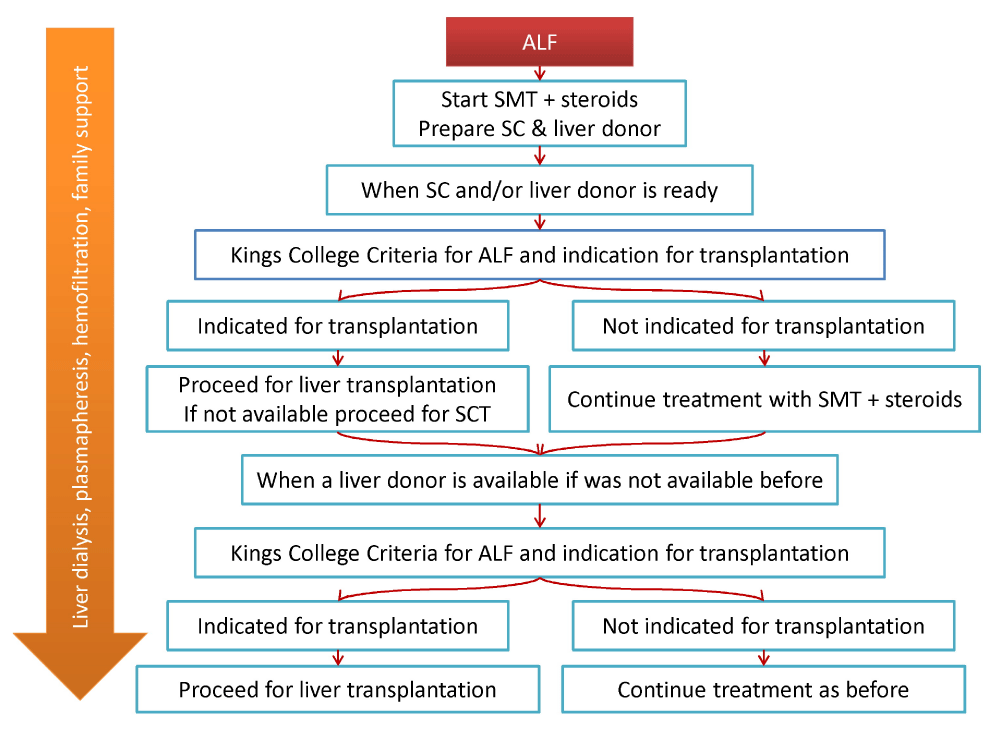
Understanding SCT’s current status in different pediatric liver disorders, the outcome, obstacles, and possible solutions could be the basis for well-designed trials for SCT in ALF in children. We recommend that when a child presents with ALF, a stepwise therapy should be rapidly applied. The first step is the introduction of steroids from the first day beside the SMT. Then rapidly preparing allogeneic or autologous SCs hand in hand with a liver donor preparation, living-related or cadaveric according to the country policy in organ transplant. When the SCs and/ or the liver donor is ready, the patient status is evaluated, if significantly improving, and the Kings’ criteria discourage LT, continue on the previous regimen. If not improving and the Kings’ criteria suggest high mortality and indication for transplantation, proceed for LT if donor is available; otherwise, SCs are infused. When a liver donor is ready (if was not prepared before), reassess the patient. If improving, continue on the previous lines; if not, proceed for the third step, LT. We hypothesize that this approach could considerably fill the gap for the many lost cases (Figure 2).
Figure 2: Algorithm for the proposed stepwise approach for the management of acute liver failure in children.
SCT in ALF could be lifesaving. Future clinical trials in pediatrics to optimize the source, administration route, and frequencies are worthy.
- Zakaria HM, Salem TA, El-Araby HA, Salama RM, Elbadry DY, et al. Steroid therapy in children with fulminant hepatitis A. J Viral Hepat. 2018; 25: 853-859. PubMed: https://pubmed.ncbi.nlm.nih.gov/29397017/
- Robert H. Squires, Alonso EM. Acute liver failure in children. In: Frederick J. Suchy, Ronald J. Sokol, Balistreri WF, editors. Liver Disease in Children. United Kingdom Bell and Bain Ltd. 2014; 32-50.
- Zhang S, Yang Y, Fan L, Zhang F, Li L. The clinical application of mesenchymal stem cells in liver disease: the current situation and potential future. Ann Transl Med. 2020; 8: 565. PubMed: https://pubmed.ncbi.nlm.nih.gov/32775366/
- Majhail NS, Farnia SH, Carpenter PA, Champlin RE, Crawford S, et al. Indications for Autologous and Allogeneic Hematopoietic Cell Transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biology of blood and marrow transplantation. Biol Blood Marrow Transplant. 2015; 21: 1863-1869. PubMed: https://pubmed.ncbi.nlm.nih.gov/26256941/
- Sureda A, Bader P, Cesaro S, Dreger P, Duarte RF, et al. Indications for allo- and auto-SCT for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2015. Bone Marrow Transplant. 2015; 50: 1037-1056. PubMed: https://pubmed.ncbi.nlm.nih.gov/25798672/
- Tan EY, Boelens JJ, Jones SA, Wynn RF. Hematopoietic Stem Cell Transplantation in Inborn Errors of Metabolism. Front Pediatr. 2019; 7: 433. PubMed: https://pubmed.ncbi.nlm.nih.gov/31709204/
- Wachowiak J, Labopin M, Miano M, Chybicka A, Stary J, et al. Haematopoietic stem cell transplantation in children in eastern European countries 1985-2004: development, recent activity and role of the EBMT/ESH Outreach Programme. Bone Marrow Transplant. 2008; 41: S112-117. PubMed: https://pubmed.ncbi.nlm.nih.gov/18545232/
- de Miguel MP, Prieto I, Moratilla A, Arias J, Aller M. Mesenchymal stem cells for liver regeneration in liver failure: from experimental models to clinical trials. Stem Cells Int. 2019; 2019.
- Adiwinata Pawitan J. Exploring the most promising stem cell therapy in liver failure: a systematic review. Stem Cells Int. 2019; 2019.
- Yuan S, Jiang T, Sun L, Zheng R, Ahat N, et al. The role of bone marrow mesenchymal stem cells in the treatment of acute liver failure. BioMed Res Int. 2013; 2013: 251846. PubMed: https://pubmed.ncbi.nlm.nih.gov/24312909/
- Zheng S, Yang J, Tang Y, Shao Q, Guo L, Liu Q. Transplantation of umbilical cord mesenchymal stem cells via different routes in rats with acute liver failure. Int J Clin Exp Pathol. 2015; 8: 15854-15862. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4730069/
- Huang B, Cheng X, Wang H, Huang W, la Ga Hu Z, et al. Mesenchymal stem cells and their secreted molecules predominantly ameliorate fulminant hepatic failure and chronic liver fibrosis in mice respectively. J Transl Med. 2016; 14: 45. PubMed: https://pubmed.ncbi.nlm.nih.gov/26861623/
- Shi M, Zhang Z, Xu R, Lin H, Fu J, et al. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012; 1: 725-731. PubMed: https://pubmed.ncbi.nlm.nih.gov/23197664/
- Li YH, Xu Y, Wu HM, Yang J, Yang LH, et al. Umbilical Cord-Derived Mesenchymal Stem Cell Transplantation in Hepatitis B Virus Related Acute-on-Chronic Liver Failure Treated with Plasma Exchange and Entecavir: a 24-Month Prospective Study. Stem Cell Rev Rep. 2016; 12: 645-653. PubMed: https://pubmed.ncbi.nlm.nih.gov/27687792/
- Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY, et al. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology. 2017; 66: 209-219. PubMed: https://pubmed.ncbi.nlm.nih.gov/28370357/
- Zhong YS, Lin N, Deng MH, Zhang FC, Tang ZF, et al. Deficient proliferation of bone marrow-derived mesenchymal stem cells in patients with chronic hepatitis B viral infections and cirrhosis of the liver. Dig Dis Sci. 2010; 55: 438-445. PubMed: https://pubmed.ncbi.nlm.nih.gov/19242797/
- Tang XP, Zhang M, Yang X, Chen LM, Zeng Y. Differentiation of human umbilical cord blood stem cells into hepatocytes in vivo and in vitro. World J Gastroenterol. 2006; 12: 4014. PubMed: https://pubmed.ncbi.nlm.nih.gov/16810750/
- Lorenzini S, Isidori A, Catani L, Gramenzi A, Talarico S, et al. Stem cell mobilization and collection in patients with liver cirrhosis. Aliment Pharmacol Ther. 2008; 27: 932-939. PubMed: https://pubmed.ncbi.nlm.nih.gov/18315586/
- Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009; 30: 204-213. PubMed: https://pubmed.ncbi.nlm.nih.gov/19366754/
- Amer MEM, El-Sayed SZ, Abou El-Kheir W, Gabr H, Gomaa AA, et al. Clinical and laboratory evaluation of patients with end-stage liver cell failure injected with bone marrow-derived hepatocyte-like cells. Eur J Gastroenterol Hepatol. 2011; 23: 936-941. PubMed: https://pubmed.ncbi.nlm.nih.gov/21900788/
- El-Ansary M, Mogawer S, Abdel-Aziz I, Abdel-Hamid S. Phase I Trial: Mesenchymal Stem Cells Transplantation in End Stage Liver Disease. J Am Sci. 2010; 6: 135-144.
- Galipeau J, Sensébé L. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities. Cell Stem Cell. 2018; 22: 824-833. PubMed: https://pubmed.ncbi.nlm.nih.gov/29859173/
- Kim JK, Kim SJ, Kim Y, Chung YE, Park YN, et al. Long-term follow-up of patients after autologous bone marrow cell infusion for decompensated liver cirrhosis. Cell Transplant. 2017; 26: 1059-1066. PubMed: https://pubmed.ncbi.nlm.nih.gov/28120743/
- Kang SH, Kim MY, Eom YW, Baik SK. Mesenchymal Stem Cells for the Treatment of Liver Disease: Present and Perspectives. Gut Liver. 2020; 14: 306. PubMed: https://pubmed.ncbi.nlm.nih.gov/31581387/
- Peng L, Xie Dy, Lin BL, Liu J, Zhu Hp, et al. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short‐term and long‐term outcomes. Hepatology. 2011; 54: 820-828. PubMed: https://pubmed.ncbi.nlm.nih.gov/21608000/