More Information
Submitted: 27 March 2020 | Approved: 06 April 2020 | Published: 07 April 2020
How to cite this article: Fayadh MH, Awadh S, El Kiwisney L, Quadri AH, Shetty PK, et al. Hyperparathyroidism in celiac disease: A case study from UAE. Ann Clin Gastroenterol Hepatol. 2020; 4: 011-014.
DOI: 10.29328/journal.acgh.1001016
Copyright License: © 2020 Fayadh MH, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Celiac disease; Hyperparathyroidism; UAE
Hyperparathyroidism in celiac disease: A case study from UAE
Makki H Fayadh*, Salim Awadh, Loai El Kiwisney, Abdul Hadi Quadri, Prasad K Shetty and Mervat Naguib
Consultant, Physicians & Gastroenterologists, Advanced Center for Daycare Surgery, LLC, Abu Dhabi, UAE
*Address for Correspondence: Sarhani Asmae, EFD-HGE, Universal Hospital Ibn Sina - UM5S, Rabat, Morocco, Tel: 00212648878660; Email: [email protected]
Celiac disease affects 1% of the world population; however it is under diagnosed in UAE. The disease has many clinical manifestations, ranging from severe malabsorption to minimally symptomatic or non-symptomatic presentation. Hypocalcaemia is a common finding in celiac disease and could be the only presentation of the disease; however hypercalcemia has been previously reported in patients with celiac disease either due to primary hyperparathyroidism or tertiary hyperparathyroidism due to prolonged hypocalcaemia. A normal calcium level on the other hand in patients with untreated celiac disease who also have primary hyperparathyroidism can be due to interplay of these two conditions and may delay the diagnosis of primary Hyperparathyroidism. We report the very first case from our practice in UAE with untreated celiac disease and normal calcium level at presentation, where a diagnosis of primary hyperparathyroidism was not entertained initially. Patient went on gluten free diet which then caused normalization of intestinal abnormalities and likely calcium absorption manifesting as hypercalcemia on subsequent labs. This led to further work up and finally the diagnosis of Primary hyperparathyroidism due to parathyroid adenoma.
Celiac disease (CD) is the most common autoimmune disorder induced by gluten ingestion which is found in wheat, oats, rye, barley and other grains. It occurs in subjects with genetic predisposition namely, HLA-DQ2 and HLA-DQ8 haplotypes and characterize by high titers of serum antigliadin and antitransglutaminase antibodies that improves after removal of gluten from the diet [1]. Generally CD occurs between 6 and 18 months of age, after the introduction of gluten containing diet [2], however it can present at any age and the peak of onset of adult CD is during the 4th and 5th decades in females and 5th and 6th decades in males [3].
CD is most missed disease, was once thought to be very rare and affect only European population but current data showed worldwide prevalence of about 1% of the general population [4]. The time taken to make the diagnosis may go to more than 10 years [5]. Studies in UAE showed positive celiac serology in 2.3% of asymptomatic pre marriage individuals [6]. Diagnosis rates are increasing, and this seems to be due to a true rise in incidence rather than increased awareness and detection [5].
CD is characterized by histopathological changes in the small intestine that includes; decreased enterocyte height, crypt hyperplasia, villous atrophy, increased intraepithelial T lymphocytes [7]. These changes in the intestinal mucosa lead to malabsorption and development of a number of nutritional deficiencies such as anemia, hypoproteinemia, and hypocalcemia.
The presentation of CD is greatly heterogeneous, depending on the patient’s age, the duration and severity of disease. The classic presentation is growth failure, malnutrition, diarrhea, abdominal pain and distension within the first 2 years of life. In contrast, many patients with celiac disease present at a later age with subtle symptoms such as diarrhea, unexplained anemia and osteoporosis and the diagnosis may be delayed [8]. Therefore, serological testing should be done in subjects in whom the diagnosis of CD is considered, such as those with vitamin or mineral deficiencies, osteoporosis/osteopenia, infertility or other clinical symptoms.
Endomysial (EMA) and tissue transglutaminase (TG IgA) autoantibodies are the most sensitive and specific for the diagnosis of CD [9]. Also, antibodies against deamidated gliadin peptides (DGP) that reflect immunity against the dietary antigen have been identified [10]. Diagnosis of CD is usually suggested by the presence of TG autoantibodies, but confirmed by biopsy of the small bowel by upper intestinal endoscopy [11].
Hypocalcemia has been reported in 5.7% of patients with CD as a result of vitamin D malabsorption or calcium loss due to binding to unabsorbed fatty acids [12]. Primary hyperparathyroidism (PHPT), usually owing to parathyroid adenoma, is associated with multiple complications such as osteoporosis, bone fractures and renal stones. Most of cases are asymptomatic and incidental hypercalcemia is the initial finding in the diagnosis [13]. To our knowledge no reported cases in UAE with celiac disease and PHPT.
A 40 year old Iraqi female diagnosed as celiac disease since 1997 and was on gluten free diet that was stopped in 2008, presented in 2019 with severe acid reflux, gaseous distention, polyuria, bone pain and sever osteopenia as detected by DEXA scan. Her laboratory tests at that time showed iron deficiency anemia, strongly positive celiac serology, and slightly elevated calcium (Table 1).
| Table 1: Laboratory data before and 6 months after starting gluten free diet. | ||
| Laboratory data | Before starting gluten free diet |
6 months after starting Gluten free diet |
| Hemoglobin (g/d) (12 - 15) | 9.3 | 12.2 |
| Iron (ug/dL) (50 - 70) | 23.00 | 59.1 |
| Calcium (mg/dL) (8.4 - 10.2) | 10.4 | 14.2 |
| PTH | - | 241.40 |
| Vitamin D (ng/ml) (> 30) | - | 35.73 |
| Magnesium (mg/dL) | 2.29 | 1.9 |
| TSH (uIU/mL ) | 0.88 | 1.24 |
| Vitamin B12 (pg/ml) (187 - 883) | 210.00 | 356.6 |
| Anti-Tissue Transglutaminase IgA (AU/ml) (<12) | Positive (200) | Positive (29.0) |
| Transglutaminase IgG(AU/ml) (< 12) | Positive (100) | Equivocal (13.2) |
| Serum Deamidated Gliadin IgG (u/mL) (< 10) | Positive (100) | Negative (6.4) |
| Stool Occult Blood (NEGATIVE) | NEGATIVE | - |
| CALPROTECTIN (ug/g) (< 50) | 9.10 | - |
| PTH: Parathyroid Hormone Intact; TSH: Thyroid Stimulating Hormone | ||
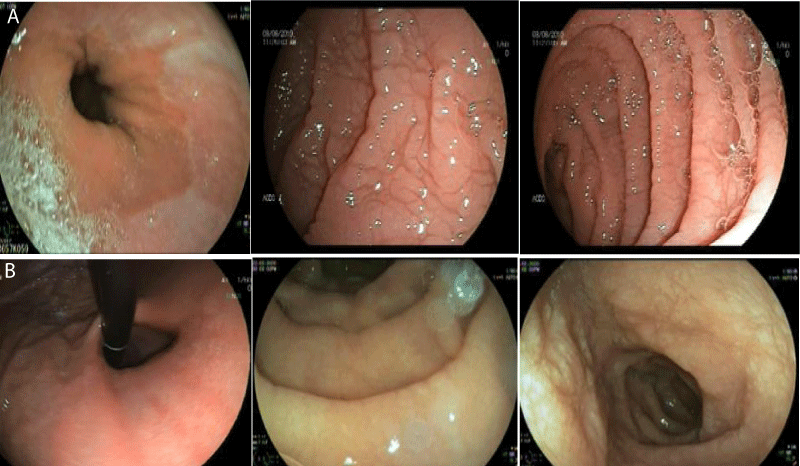
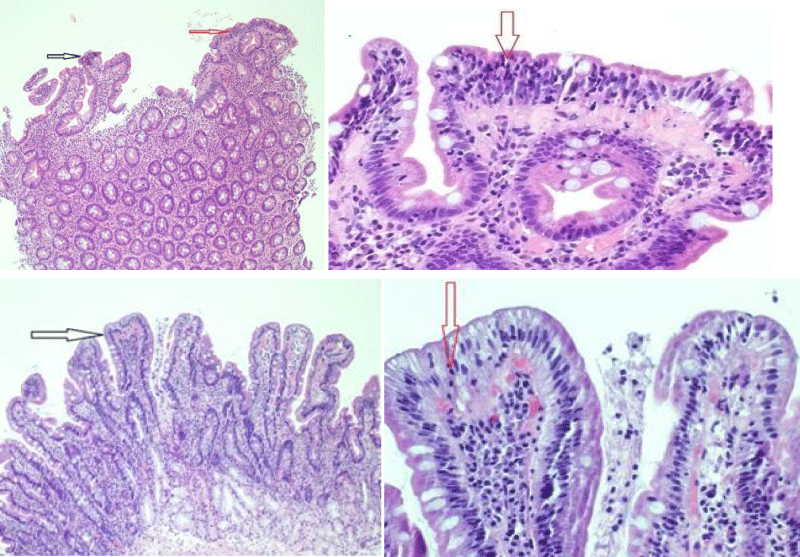
Gastroscopy Augest 2019 showed small hiatus hernia with lax cardia erythema stomach and thin serrated duodenal folds (Figure 1A). Duodenal biopsy showed CD, Type IIIB (modified marsh classification) (Figure 2A). Patient came back for a follow up after six months of gluten free diet, when her symptoms were significantly improved with weight gain and rise in Hb (Table 1). Gastroscopy was repeated that showed hiatus hernia with erosion and mild serration of duodenal folds with marked improvement in duodenal biopsy compared to the last one (Figure 1B and 2B).
Figure 1A,B: Gastroscopy in Augest 2019 showed small hiatal hernia with lax cardia erythema stomach and thin serrated duodenal folds (1A). Repeat gastroscopy in February 2020 after 6 months of gluten free diet showed small hiatus hernia with erosion, mild serration of duodenal folds and improved compared to last one.
Her laboratory tests in February 2020 showed improved celiac serology iron deficiency anemia. At that time she showed severe hypercalcemia (14.2 mg/dL normal range 8.6 -10.0 mg/dl), and elevated Parathyroid Hormone Intact (PTH); (241.40 pg/ml normal range 15-65).
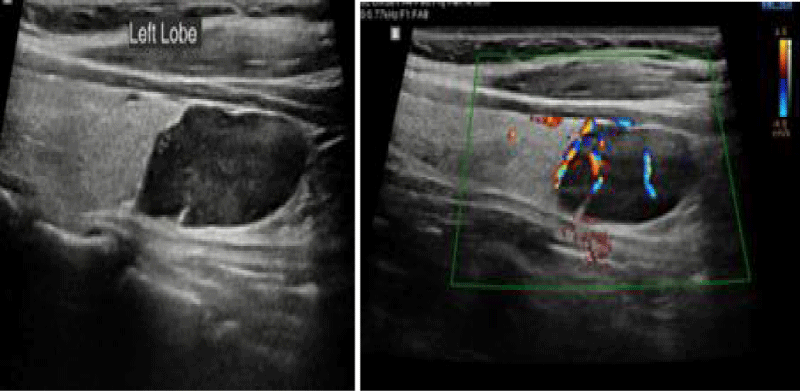
Ultrasound of neck in February 2020 showed hypo echoic lesion in the mid and lower left thyroid lobe measuring 12 x 27 mm in diameters suggestive of parathyroid adenoma (Figure 3). The patient received one injection of Denosumab 60 mg, and the calcium level decreased to 9.4 mg/dl. The patient has been scheduled to have parathyroidectomy.
Figure 2: Shows marked villous atrophy (black arrow) and significant intraepithelial lymphocytes 50-60/ 100 enterocytes (red arrow).Repeated biopsy after 6 months of gluten free diet showed Celiac Disease (Type 0 modified marsh classification) with preserved villus architecture (black arrow).
Figure 3: MNeck ultra sound in February 2020 showed hypo echoic mass in the mid and lower left thyroid lobe measuring 12 x 27 mm in diameters suggestive of parathyroid lesion.
In our experience in Iraq and UAE we came across a variety of presentations of CD ranging from reflux disease, irritable bowel syndrome, anemia, recurrent stomatitis, infertility, elevated liver enzymes, short stature, obesity, association with inflammatory bowel diseases and they missed because of lack of awareness by the health care workers. Hypocalcemia is more common than hypercalcemia in patients with untreated celiac disease. A normal or near normal calcium level is a more common finding then either hypo or hypercalcemia. The effect of gluten free diet on ionized calcium has been documented [12].
CD is typically accompanied by vitamin D deficiency and calcium malabsorption, both of them cause secondary hyperparathyroidism [13]. Although the assemblage of CD and elevated PTH levels is anticipated, the association of CD with hypercalcemia and subsequent diagnosis of PHPT is not. After prior scarce reports of the coexistence of these conditions, one study reported the prevalence of primary hyperparathyroidism could reach up to 2.3% in patients with CD [14]. The abnormal absorption of calcium and Vitamin D from intestine due to CD may spuriously lower the Calcium, masking the effect of Primary Hyperparathyroidism, hence delaying the diagnosis. Gluten free diet correcting the intestinal abnormalities leading to normalization of the calcium absorption may then unmask the hypercalcemia caused by the primary hyperparathyroidism.
Our case is the first reported case in UAE with celiac disease and primary hyperparathyroidism. It is also the first reported case showing the effect of CD on calcium levels, affecting the diagnosis of primary hyperparathyroidism. The diagnosis of PHPT in patient with celiac disease could be challenging as CD and PHPT share many overlapping, non‐specific symptoms such as fatigue, depression, bone pain and abdominal pain that may delay the clinical diagnosis [15]. Usually there is 1-4 years delay in diagnosis of hyperparathyroidism in patients with CD as elevated PTH usually justified by the low vitamin D levels in patients with CD [16]. In our case the patient had bone pain and osteopenia that was initially linked to malabsorption and vitamin D deficiency. Furthermore, when the patient was not compliant on gluten free diet her calcium level was 10.4 mg/dl, however her calcium level markedly raised up to 14.2 mg/dl after 6 months of gluten free diet and this was corresponding to improvement of intestinal mucosa leading to increased absorption of calcium and unmasking of hyperparathyroidism.This emphasizes the need to look for hyperparathyroidism in patients with CD and normal calcium level and persistent symptoms despite adherence to gluten free diet.
Tertiary hyperparathyroidism may develop in patients with longstanding hypocalcemia as in undiagnosed cases of CD and when they start treatment the hypercalcemia becomes apparent [17]. In our case tertiary hyperparathyroidism is a remote possibility as the patient initially has no hypocalcaemia. Furthermore, hyperplasia of the parathyroid glands is the common morphological feature and the development of adenoma in tertiary hyperparathyroidism account only for 5% of cases [18,19], and in our patient there was large adenoma rather than hyperplasia.
• Celiac disease and hyperparathyroism can be associated
• Awareness is needed as both celiac disease and hyperparathyroidism are under recognized
• More studies are required to clarify the relationship between coeliac disease and hyperparathyroidism
- Murch S, Jenkins H, Auth M. Joint BSPGHAN and Coeliac UK guidelines for the diagnosis and management of coeliac disease in children. Arch Dis Child. 2013; 98: 806-811. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23986560
- Tully MA. Pediatric celiac disease. Gastroenterol Nurs. 2008; 31: 132-140.
- Corazza GR, Gasbarrini G. Celiac disease in adults. Bailliere's Clinical Gastroenterol. 1995; 9: 329-350. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/7549030
- Kamin DS, Furuta GT. The iceberg cometh: establishing the prevalence of celiac disease in the United States and Finland. Gastroenterology. 2004; 126: 359-361.
- Green PH, Cellier C. Celiac disease. N Engl J Med. 2007; 357: 1731-1743. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17960014
- Abu-Zeid YA, Jasem WS, Lebwohl B. Seroprevalence of celiac disease among United Arab Emirates healthy adult nationals: a gender disparity. World J Gastroenterol. 2014; 20: 15830-15836.
- Oberhuber G. Histopathology of celiac disease. Biomed Pharmacother. 2000; 54: 368-372. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10989975
- Freeman HJ, Chopra A, Clandinin MT, Thomson AB. Recent advances in celiac disease. World J Gastroenterol. 2011; 17: 2259-2272. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21633592
- Farrell RJ, Kelly CP. Sprue. N Engl J Med. 2002; 346: 180-188.
- Liu E, Li M, Emery L. Natural history of antibodies to deamidated gliadin peptides and transglutaminase in early childhood celiac disease. J Pediatr Gastroenterol Nutr. 2007; 45: 293-300. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17873740
- Barker JM, Liu E. Celiac disease: pathophysiology, clinical manifestations, and associated autoimmune conditions. Adv Pediatr. 2008; 55: 349-365.
- Resistance to Vitamin D Treatment as an Indication of Celiac Disease in a Patient with Primary Hypoparathyroidism. 2009.
- Posthumus L, Al-Toma A. Duodenal histopathology and laboratory deficiencies related to bone metabolism in coeliac disease. Eur J Gastroenterol Hepatol. 2017; 29: 897-903. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28452813
- Bilezikian JP, Silverberg SJ. Clinical spectrum of primary hyperparathyroidism. Rev Endocr Metab Disord. 2000; 1: 237-245.
- Ludvigsson JF, Kämpe O, Lebwohl B. Primary hyperparathyroidism and celiac disease: a population-based cohort study. J Clin Endocrinol Metab. 2012; 97: 897-904. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/22238405
- Maida MJ, Praveen E, Crimmins SR. Coeliac disease and primary hyperparathyroidism: an association? Postgrad Med J. 2006; 82: 833-835.
- Alzahrani AS, Al Sheef M. Severe primary hyperparathyroidism masked by asymptomatic celiac disease. Endocr Pract. 2008; 14: 347-350.
- Anaforoglu I, Ersoy K, Algun E. Parathyroid adenoma with coeliac disease: primary or quaternary hyperparathyroidism? Endokrynol Pol. 2012; 63: 56-58.
- Krause MW, Hedinger CE. Pathologic study of parathyroid glands in tertiary hyperparathyroidism. Hum Pathol. 1985; 16772-16784. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/4018775