More Information
Submitted: 21 March 2020 | Approved: 03 April 2020 | Published: 06 April 2020
How to cite this article: Yetisir F, Güzel K. Laparoscopic anterior transgastric cystogastrostomy for the treatment of pancreatic pseudocysts. Ann Clin Gastroenterol Hepatol. 2020; 4: 006-010.
DOI: 10.29328/journal.acgh.1001015
Copyright License: © 2020 Yetisir F, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Pancreatic pseudocyst; Laparoscopic cystogastrostomy; Internal drainage of pseudocyst; Versa-lifter
Laparoscopic anterior transgastric cystogastrostomy for the treatment of pancreatic pseudocysts
Fahri Yetisir1* and Kerim Güzel2
1AVM Medical Park, Ankara Private Hospital, Yüksek İhtisas University, General Surgery Department, Ankara, Turkey
2Department of General Surgery , Near East University, Faculty Of Medicine, 99138 Nicosia, TRNC, Cyprus
*Address for Correspondence: Fahri Yetisir, Ankara Medisis Private Hospital General Surgery Department, Ankara, Turkey, Tel: 05362974888; Email: drfahriyetisir@hotmail.com
Introduction: Pancreatic pseudocysts (PPs) are mostly delayed complications of acute or chronic pancreatitis and trauma. Pancreatic pseudocysts are usually managed by supportive medical treatment without surgical procedure. All the surgical interventions (percutaneous, endoscopic or surgical approaches) are based on the location, size, symptoms, complications of the pancreatic pseudocyst and medical condition of the patients. Recently, laparoscopic cystogastrostomy has become most appropriate approach especially for retrogastric pancreatic pseudocysts. In this study, we would like to report results of laparoscopic anterior transgastric cystogastrostomy by using linear articulated endo GIA stapler (Covidien medium thick purple) and versa-lifter (versa lifter®, laparoscopic retractor, manufactured by protomedlabs, France) in 14 pancreatic pseudocysts patients.
Methods: We retrospectively analyzed data of patients with pancreatic pseudocysts treated by laparoscopic anterior transgastric cystogastrostomy from September 2010 to October 2014. All of the patients were controlled for the recurrence of pancreatic pseudocysts in February 2017.
Results: 14 patients with pancreatic pseudocysts were managed by laparoscopic anterior transgastric cysto-gastrostomy. Conversion was performed in only one patient (7%). There were no symptoms and signs of recurrence of pancreatic pseudocyst during on average 43.6 months follow up time.
Conclusion: Laparoscopic cystogastrostomy by using articulated linear endo-GIA stapler and versa-lifter is a safe and effective method for management of appropriate retro-gastric pancreatic pseudocysts.
The first description of pancreatic pseudocyst (PP) was reported at 1761 [1]. PP is a localized fluid collection that may contain pancreatic fluid, necrotic debris and blood. In some cases, PPs may be superinfected and abscess formation may occur. Imaging of PP shows fluid surrounded by a well-defined wall containing no solid material. This surrounding wall is occurred from fibrous tissue that is not lined by real epithelium [2]. PPs are connected with the duct system of pancreas, either as a direct communication or indirectly via the pancreatic parenchyma. They are usually developed by pancreatic ductal disruption following increased pancreatic ductal pressure, either due to stenosis, calculi or protein plugs obstructing the main pancreatic ductal system, or as a result of pancreatic necrosis following an attack of acute or chronic pancreatitis and trauma [3,4]. Frequency of PPs occurrence after acute and chronic pancreatitis is 5% - 12% and 30% - 40% respectively [5-7].
There are a lot of different management modalities for PPs. In most cases, patients with PPs are recovered well with medical supportive treatment without any intervention [8]. There are two main indications for invasive interventions; first main indication is the presence of symptoms and/or complications (infection, bleeding, gastric outlet or biliary obstruction) of the PP [9]. Second main indication is persistent PP’s diameter which is greater than 6 cm after 6 weeks duration. There are especially three different interventions (percutaneous, endoscopic or surgical) in the management of PPs [10]. Endoscopic internal drainage of PPs has become more common in the last decade for proper cases. Total excision, external and internal drainage of PPs could be done by open or laparoscopic surgical treatment approaches. In recent years, internal drainage is the most accepted and applied surgical approach with less mortality ratio and morbidity. It can be done as a cystogastrostomy, cystojejunostomy and cystoduodenostomy [11]. Since 1994, PPs have been managed laparoscopically [12]. Laparoscopic anterior cystogastrostomy is the most commonly preferred procedure for retrogastric PPs [8,9,13]. In the last decade, especially minimally invasive approach is increasingly used in the management of PPs.
This study was carried out to document our experience with laparoscopic anterior transgastric cystogastrostomy for the treatment of patients with PPs by using articulated linear endo GIA stapler (Covidien medium thick purple) and versa-lifter(versa lifter®, laparoscopic retractor, manufactured by protomedlabs, France).
We analyzed data of patients with PP treated by laparoscopic anterior transgastric cystogastrostomy retrospectively. PP was diagnosed mainly by clinical features and imaging techniques (ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), endo US).Size of PPs was measured with US or CT. Hematological and biochemical parameters were evaluated in all patients. The data included age, gender, body mass index (BMI), diameter of cyst, etiology of PPs, waiting time interval for maturation of PP (time between initial presentation of PP and operation), operation time, length of hospital stay, follow up time and outcome of management were recorded. Written informed consent was taken from all patients.
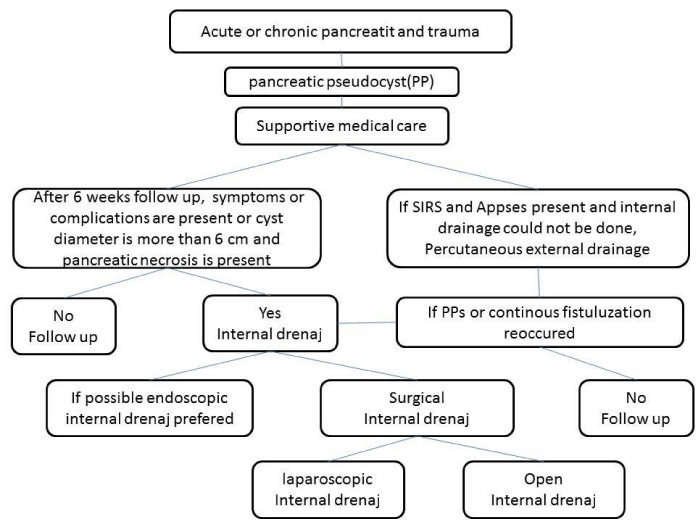
Treatment algorithm of PPs
Management of all the patients with PPs was initiated with supportive medical care (intravenous fluid, analgesic and antiemetic medications). During preoperative period, fluid and electrolyte imbalance of the patients were corrected. Antibiotherapy was started in the cases of abscess formation in the PP. Intervention to the PP was performed in the presence of the symptoms or complications of PPs and persistent PPs size more than 6 cm after 6 weeks duration. Percutaneous drainage (external drainage) of infected PP was preferred, if only the internal drainage could not be performed (patient could not undergo any type of operation due to hemodynamic instability or endoscopic internal drainage could not be applied). Internal drainage was applied to all of the remaining patients. Minimal invasive approach was preferred in suitable cases; endoscopic internal drainage was preferred in some suitable cases as a first choice. If endoscopic internal drainage could not be performed or after failure and recurrence of endoscopic intervention, laparoscopic approach was preferred secondly, open surgery was preferred as a last choice (Figure 1).
Figure 1: Flow chart of treatment algorithm of pancreatic pseudocysts.
Surgical technique of laparoscopic cystogastrostomy
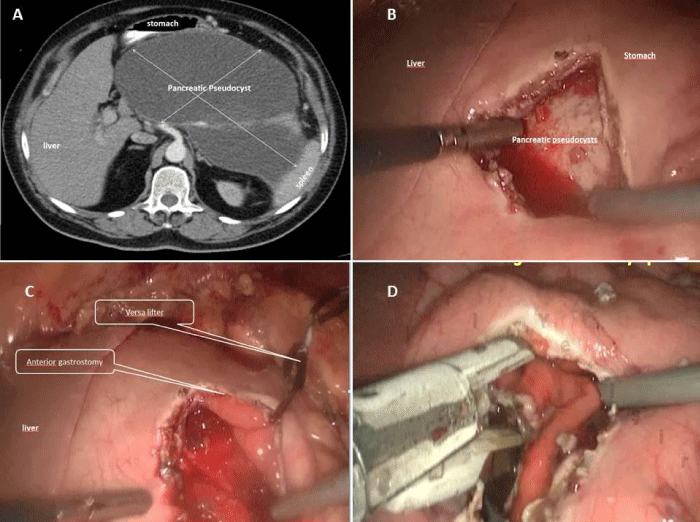
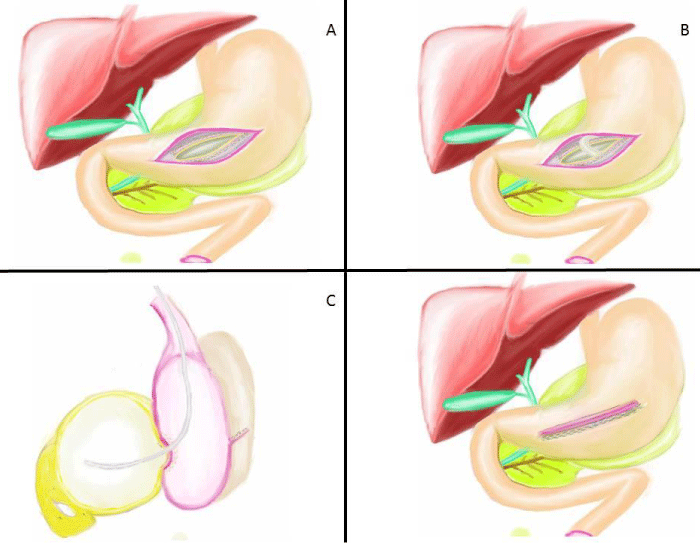
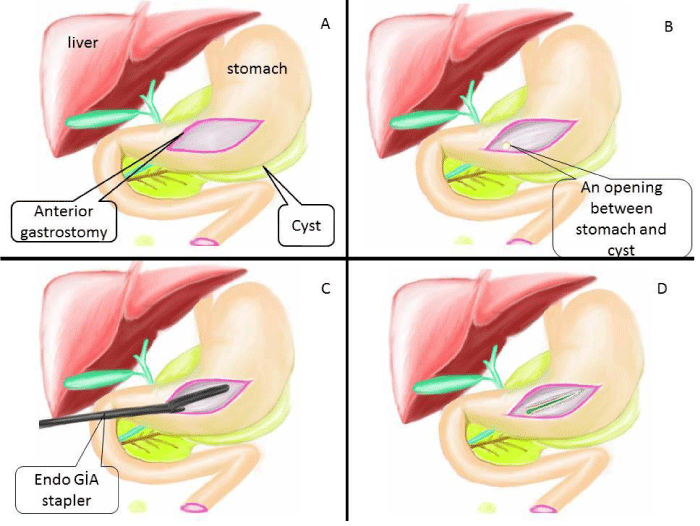
All patients were operated under general anesthesia and patients were positioned in modified semi-lithotomy position, with the operating surgeon standing between the legs of the patient, the camera surgeon on the right side of the patient and the assistant surgeon standing on the left side of the patient. The monitor was placed at the head end of the patient. A 5 mm port was placed sub-umbilical for 5 mm 30oC angle scope and after CO2 insufflation, camera was entered into the abdomen. Under vision, another 5-mm port was placed in the left subcostal area and a 12 mm port was placed in the right subcostal area. If patient had gallstone, cholecystectomy was performed firstly. For cholecystectomy operation, one more 5 mm trocar was entered from right subcostal region. During the exploration of abdominal cavity, the edge of cyst was tried to be defined with the help of the previous CT imaging also (Figure 2A). Sometimes gastro-colic ligament was opened by using harmonic scalpel and the mass of retrogastric PP was visualized or felt by touching with the grasper. A 5 cm anterior gastrostomy was made by the harmonic scalpel (Ethicon Endosurgery, Cincinnati, USA) at the maximal displacement site of the stomach by the PP (Figure 2B, 3A). A better view of operating field was obtained by suspending one edge of anterior gastrostomy by using versa-lifter (Figure 2C). The mass of PPs on posterior side of stomach was visualized by entering into the stomach. Preliminary aspiration of the cysts was done with a size-21 G needle to exclude pseudoaneurysm. Purulent aspirates were sent for bacterial cultures. Then, by using the harmonic scalpel or electrocautery, approximately 1 cm in size opening between the adherent posterior gastric wall and anterior pseudocyst wall was created (Figure 3B). Fluid contents were aspirated and a piece of sample from pseudocyst wall was obtained for pathological analysis. One or two linear endo GIA stapler was used to create cystogastrostomy (Figure 2D, 3C). After that, all the debris and necrotic material was taken out and the cyst cavity was irrigated with saline (Figure 3D, 4A). Nasogastric tube was placed inside the cyst and stomach (Figure 4B,4C). The anterior gastrostomy was closed using linear endo GIA staplers (Figure 4D). The peritoneal cavity was irrigated with saline and aspirated and finally a drainage tube was placed. Operation was terminated after taking out all the trocars. All the operation are illustrated in figures.
Follow up
The nasogastric tube was removed and enteral nutrition was started on the 2nd - 4th postoperative day. They were controlled at postoperative 1st, 3rd and 6th month for signs and symptoms related to PPs or recurrence. Last controls of all patients were made in February 2017. Symptoms of PP were questioned and abdominal US results were evaluated. If there was any conflict, CT was used for clarification.
Figure 2: Computed tomography (CT) image of PP and all surgical steps of laparoscopic anterior transgastric cystogastrostomy operation were seen: A) A giant pancreatic pseudocyst. B) Anterior bulging of stomach due to PP after anterior gastrostomy. C) The versa lifter was applied to one edge of gastrostomy D) Cystogastrostomy was created by a linear endo GIA stapler inside the stomach.
Figure 3: Illustration of surgical step part I; A) First, A 5 cm anterior gastrostomy at the maximal displacement side of the stomach by the harmonic scalpel. B) Then, approximately 1 cm in size opening was created between the adherent posterior gastric wall and anterior pseudocyst wall by using electrocautery. C) Cystogastrostomy was created by using one or two linear endo GIA stapler. D) Finally, cystogastrostomy formation was seen.
Figure 4: Illustration of surgical step part II; A) All the debris and necrotic material was taken out and cyst was irrigated with saline. B) Nasogastric tube was placed inside cyst. C) Lateral view of this operation (Nasogastric tube was replaced inside cyst) D) Anterior gastrostomy opening was closed by linear endo GIA stapler.
Fourteen patients with PPs underwent laparoscopic anterior transgastric cystogastrostomy operation from September 2010 to October 20014. There were 8 male and 6 female patients. Mean age was 40.5 ± 12.1(min-max: 23-65) years. Mean body mass index (BMI) was 27.5 ± 4.7 kg/m2 (Table 1). American Society of Anesthesiologists (ASA) scores distribution of these patients were as follows; 2 of them had ASA I, 8 of them had ASA II and 4 of them had ASA III. 8 of the patients had gall stone disease and cholecystectomy was also performed during the cystogastrostomy operation. 4 of them had prior history of alcohol ingestion and one of them had dyslipidemia disorder and etiology was not apparent in 2 patients. Endoscopic drainage could not be performed due to technical difficulties in 5 patients, 3 patients had PP recurrence after endoscopic drainage, and endoscopic drainage could not be performed successfully in 6 patients. Mean length of waiting time interval was 6.6 ± 2.8 (min-max: 3-12) months. Sizes of all PPs were demonstrated in table 1. Mean duration of operation was 96.1 ± 35.5 (min-max: 50-170) minutes. Mean length of operation without cholecystectomy and with cholecystectomy were 63.5 ± 17.9 and 120.6 ± 22.9 minutes respectively. Additional cholecystectomy significantly prolonged operation duration (p < 0.001). Two patients (14%) had multiple PPs. One (7%) patient had infected PP and underwent external drainage before operation. All cases were successfully operated without any significant intraoperative complication. There was only one (7%) conversion due to the difficulty of determination of PP’s border. Only 2 patients needed to stay in ICU because of the metabolic alkalosis. Mean length of hospital stay was 5.3 ± 5.2 (min-max: 3-23) days. Mean follow up period was 43.6 ± 14.5 (min-max: 29-75) months. There were no symptoms and signs of recurrent PP during this follow-up period. Incisional hernia developed on the trocar entrance side of one (7%) patient. 5 (83%) of the 6 cystogastrostomy operations without cholecystectomy were completed with 3 ports.
In this study, fourteen patients with PPs underwent laparoscopic anterior transgastric cystogastrostomy operation without complications. There was no recurrent PP during early follow-up period. 5 (83%) of the 6 cystogastrostomy operations without cholecystectomy were completed with 3 ports.
Mehta, et al. showed that 30% - 60% of PPs has been asymptomatic and spontaneously resolved after acute pancreatitis [14]. Some patients with PPs need interventions. There are lots of factors determining the route and time of intervention [15]. These factors are; location, size and persistence of the cyst, [9] maturity of the cyst wall and [10] presence of complications [15]. Most frequently seen indications for intervention of PPs are symptoms (including epigastric pain, nausea, vomiting, biliary obstruction, and duodenal obstruction), complications (including infection, hemorrhage, rupture) and more than 6 cm in diameter after 6 weeks duration [8,16]. The matured wall during operation is prerequisite for successful treatment.
Surgical interventions for management of PPs are percutaneous, endoscopic, laparoscopic and open surgical procedures. There are specific indications and contraindications for all these intervention type. If PPs have been infected and obstructed with an immature cyst wall percutaneous drainage is the procedure of choice especially for hemodynamically instable patients. However, percutaneous drainage seems to have a high risk of recurrence or development of pancreatic percutaneous fistula. It is also used in cases with poor general condition and refusal of the operation and definitive internal drainage could not be performed [9]. Furthermore, percutaneous drainage may be inadequate in many cases due to thick viscous contents [17]. Percutaneous drainage was performed to one patient due to hemodynamic instability but he was operated again by laparoscopic cystogastrostomy because of the inadequate drainage of the PP in the present study.
Although, endoscopic drainage is associated with a high rate of cyst recurrence, stent blockage, technical failure, infection, bleeding, and inadequate drainage, it is an important procedure in the management of pseudocysts, especially the cysts indenting the stomach or duodenum and in the absence of necrotic tissue [9]. In our study, 3 of the 14 patients were recurrent cases after endoscopic drainage. Aljarabah, et al. has reported that endoscopic drainage has been more suitable for chronic PPs within the head and body of the gland, whereas laparoscopic surgery is the best treatment choice in acute PPs with the complication of necrotizing pancreatitis [8]. Recently, laparoscopic surgery for the suitable PPs has become the gold standard procedure [10]. During PPs management, there were some advantages of laparoscopic approach over endoscopic approach. They include the ability to create a larger anastomosis, and easier management of complications such as hemorrhage or perforation, in addition to evaluation and debridement of the inner cyst cavity [18]. In some cases, surgery, endoscopic drainage, and percutaneous external drainage are complementary to each other rather than being conflicting alternatives [19].
Christos, et al reported that management of PPs has evolved over the years, from an aggressive approach, to a more conservative management. In fragile patients or those that cannot tolerate other modalities of treatment, percutaneous drainage can be offered with good results. Especially after the introduction of EUS, endoscopic procedures seem to be safe, effective and probably will become the preferred method of choice. Finally, the historical gold standard of surgical drainage has proven its efficacy and with the addition of laparoscopy, remains a reliable method especially in large and complicated pseudocyst [20].
Cystogastrostomy, cystoduodenostomy and cystojejunostomy can be done by open or laparoscopic way in the surgical management of PPs. Laparoscopic internal drainage can be done using anterior gastrostomy and posterior approaches [21]. While the anterior approach is easily performed, the posterior laparoscopic approach requires more sophisticated laparoscopic skill.
Only laparoscopic cystogastrostomy operation without cholecystectomy was performed in 6 patients. In 5 of these 6 patients, laparoscopy was carried out using a 3-port technique instead of 4-port by using versa-lifter. Versa-lifter for suspending one edge of gastrostomy diminished one more extra trocar requirement. By this way, two laparoscopic surgical tools were under control of surgeon. Optimal drainage of cyst cavity to stomach with large cystogastrostomy anastomosis by using linear endo GIA stapler was provided. Therefore the recurrence was diminished. All of our cases had no symptoms during 43.6 month follow-up period.
Nowadays, laparoscopic surgical drainage is still the gold standard treatment modalities for appropriate PPs with highest success and lowest recurrence rate. Laparoscopic cystogastrostomy by using linear stapler via anterior gastrostomy can be used effectively and safely for the management of the retrogatric PPs. During this operation versa-lifter works as a second assistant by diminishing one trocar entrance without any loss of surgical comfort.
- Cannon JW, Callery MP, Vollmer CM. Diagnosis and management of pancreatic pseudocysts: what is the evidence? J Am Coll Surg. 2009; 3: 385-393. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19717045
- Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis. Arch Surg. 2009; 128: 586-590. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8489394
- Sanfey H, Aguilar M, Jones RS. Pseudocysts of the pancreas, a review of 97 cases. Am Surg; 60: 661-668 4. Gumaste VV, Pitchumoni CS. (1996) Pancreatic pseudocyst. Gastroenterologist. 1994; 4: 33-43.
- Boerma D, Obertop H, Gouma DJ. Pancreatic pseudocysts in chronic pancreatitis.Surgical or interventional drainage? Ann Ital Chir. 2000; 71: 43-50. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10829523
- Maringhini A, Uomo G, Patti R, Rabitti P, Termini A, et al. Pseudocysts in acute nonalcoholic pancreatitis: incidence and natural history. Dig Dis Sci. 1999; 44: 1669-1673. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10492151
- Kourtesis G, Wilson SE, Williams RA. The clinical significance of fluid collections in acute pancreatitis. Am Surg. 1990; 56: 796-799. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/2268109
- Aljarabah M, Ammori BJ. Laparoscopic and endoscopic approaches for drainage of pancreatic pseudocysts: a systematic review of published series. Surg Endosc. 2007; 21: 1936-1944. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17717626
- Samir H, Peter VD. Pancreatic pseudocyst. World J Gastroenterol. 2009; 15: 38-47.
- Palanivelu C, Senthilkumar K, Madhankumar MV. Management of pancreatic pseudocyst in the era of laparoscopic surgery--experience from a tertiary centre. Surg Endosc. 2007; 21: 2262-2267. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/17516116
- Mori T, Abe N, Sugiyama M, Atomi Y. Laparoscopic pancreatic surgery. J Hepatobiliary Pancreat Surg. 2005; 12: 451-455.
- Frantzides CT, Ludwig KA, Redlich PN. Laparoscopic management of a pancreatic pseudocyst. J Laparoendosc Surg. 1994; 1: 55-59. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/8173114
- Hamza N, Ammori BJ. Laparoscopic drainage of pancreatic pseudocysts: a methodological approach. J Gastrointest Surg. 2010; 14: 148–155. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19789929
- Mehta R, Suvarna D, Sadasivan S, John A, Raj V, et al. Natural course of asymptomatic pancreatic pseudocyst: a prospective study. Indian J Gastroenterol. 2004; 23: 140-142. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/15213900
- Yang CC, Shin JS, Liu YT, Yueh SK, Chou DA. Management of pancreatic pseudocysts by endoscopic cystogastrostomy. J Formos Med Assoc. 1999; 98: 283-286. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10389374
- Ergül Z, Yetisir F, Hoca O, Baran İ. Massive Gastrointestinal Bleeding Due to Rupture of Splenic Artery Pseudoaneurysm Into the Pancreas Pseudocyst. Journal of Clinical Sciences & Doctor. 2005; 11: 269-272.
- Haluszka O, Campbell A, Horvath K. Endoscopic managemnt of pancreatic in pseudocyst children. Gastrointest Endosc. 2002; 55: 128-131.
- Libby ED, Taylor J, Mysh D, Schwaitzberg SD. Combined laparoendoscopiccystogastrostomy. Gastrointest Endosc.1999; 50: 416–419.
- Yin WY. The role of surgery in pancreatic pseudocyst. Hepatogastroenterology. 2005; 52: 1266-1273. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16001676
- Christos A, Ioannis P, Ioannis S, Demetrios D, Christos D. Review of management options for pancreatic pseudocysts. Transl Gastroenterol Hepatol. 2018; 3: 18. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29682625
- Oida T, Mimatsu K, Kawasaki A. Long-term outcome of laparoscopic cystogastrostomy performed using a posterior ap- proach with a stapling device. Dig Surg. 2009; 26: 110-114.
- Barragan B, Love L, Wachtel M, Griswold JA, Frezza EE. A comparison of anterior and posterior approaches for the surgical treatment of pancreatic pseudocyst using laparoscopic cystogastrostomy. J Laparoendosc Adv Surg Tech A. 2005; 15: 596-600. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16366865