More Information
Submitted: 05 November 2019 | Approved: 12 November 2019 | Published: 13 November 2019
How to cite this article: Lemoine LA, Segarra-Newnham M. Evaluation of outcomes of 8-week therapy with ledipasvir/sofosbuvir or glecaprevir/pibrentasvir in veterans with hepatitis C infection. Ann Clin Gastroenterol Hepatol. 2019; 3: 027-030.
DOI: 10.29328/journal.acgh.1001011
Copyright License: © 2019 Lemoine LA, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Evaluation of outcomes of 8-week therapy with ledipasvir/sofosbuvir or glecaprevir/pibrentasvir in veterans with hepatitis C infection
L Anri Lemoine1 and Marisel Segarra-Newnham2*
1Clinical Pharmacist, Baylor Scott & White, The Heart Hospital, Plano, TX, USA
2Infectious Diseases Clinical Pharmacy Specialist, Pharmacy Department, Veterans Affairs Medical Center (VAMC), 7305 North Military Trail West Palm Beach, FL 33410, USA
*Address for Correspondence: Marisel Segarra-Newnham, Pharm D, MPH, FCCP, BCPS, BCIDP, Infectious Diseases Clinical Pharmacy Specialist, Department of Pharmacy, Veterans Affairs Medical Center (VAMC), 7305 North Military Trail, West Palm Beach, FL 33410, USA, Tel: 561-422-8262; Email: [email protected]
Hepatitis C Virus (HCV) infection is usually treated with direct acting antivirals (DAAs) for 12 weeks. In treatment naive patients with genotype (GT) 1 infection without cirrhosis and baseline viral load < 6 million, 8 weeks of Ledipasvir/Sofosbuvir (LDV/SOF) is an option. Eight weeks with Glecaprevir/Pibrentasvir (GLE/PIB) is an option for patients with GT 1 through 6 without cirrhosis. Our objective was to evaluate achievement of Sustained Virologic Response (SVR) after 8 weeks of LDV/SOF or GLE/PIB in our HCV-infected veterans. Patients with HCV infection that received GLE/PIB or LDV/SOF for a planned 8 weeks of therapy in the past four years were reviewed (January 2015-September 2018). Treatment outcomes were evaluated through medical record review.
Two hundred sixty-five veterans were initiated on 8 weeks of therapy with either GLE/PIB or LDV/SOF. Of these, 231 (87%) were initiated on 8 weeks of LDV/SOF and 34 (13%) were initiated on 8 weeks of GLE/PIB. The majority of patients had GT 1 (93%) infection. One hundred and ninety-five veterans who completed 8 weeks of LDV/SOF and 30 veterans on GLE/PIB had follow-up viral loads. The overall SVR was 95%. Treatment with GLE/PIB resulted in a higher SVR rate (100%) compared to LDV/SOF (95%). Elderly patients had similar SVR rates. Treatment with 8 weeks of DAA is effective in our veteran population and showed an SVR rate similar to literature reports. The SVR for patients treated with 8 weeks LDV/SOF was slightly lower than the SVR for GLE/PIB; however, the GLE/PIB population was smaller.
Hepatitis C Virus (HCV) infection is a disease characterized by liver inflammation which over time can progress to liver cirrhosis and other serious complications such as liver cancer [1]. HCV exhibits high genetic diversity, characterized by various genotypes and subgroups. HCV Genotype (GT) 1 is the most prevalent worldwide, however over half of all HCV infections are comprised of other genotypes [2], which poses a challenge for vaccine develop and treatment. An estimated 3.5 million people in the United States have chronic hepatitis C, with 10%-20% developing cirrhosis over a period of 20-30 years [1].
Treatment is recommended for all patients with chronic HCV infection, except in those with short (< 12 months) life expectancies secondary to non-hepatic conditions. The goal of therapy is to achieve a Sustained Virologic Response (SVR) defined as an absence of detectable virus 12 weeks after completion of therapy. HCV treatments have progressed rapidly from poorly tolerated, moderately successful interferon-based treatments to highly effective, tolerable, fixed-dose, and completely oral treatments [3]. Direct Acting Antivirals (DAAs) are the treatment of choice with once daily regimens usually taken for three months (12 weeks) [4]. These regimens have an SVR rate above 90% regardless of genotype, however cost remains high and some patient populations remain difficult to treat [1,3].
The combination of ledipasvir and sofosbuvir (LDV/SOF) was the first commercially available once-daily combination tablet for HCV GT1 infections to receive label approval by the U.S. Food and Drug Administration (FDA) in 2014. Studies showed that noncirrhotic treatment-naïve patients with HCV GT1 infection, LDV/SOF for 8 weeks was non-inferior to 12 weeks of treatment with or without ribavirin [4]. This 8-week regimen is recommended in the AASLD guidelines for HCV GT1 treatment in non-black, HIV-negative, noncirrhotic, treatment-naive patients with an RNA count below 6 million IU/mL [4,5]. Glecaprevir/pibrentasvir (GLE/PIB) is a newer DAA approved in August 2017 for 12 weeks treatment of all HCV genotypes (1, 2, 3, 4, 5, or 6) with all stages of fibrosis. It is also label-approved for 8 weeks of therapy in noncirrhotic, treatment-naive patients for all genotypes regardless of baseline viral load [4].
Shorter treatment durations decrease costs and have potential for better adherence; therefore, these regimens are preferred when indicated. HCV genotype, disease progression, and special patient populations can all influence the determination of optimal duration of therapy. A recent study of 8 weeks of therapy with LDV/SOF found that SVR12 was achieved in 97% of treatment-naïve HCV GT1 patients overall, but the SVR12 rates were lower (93%) in patients with stage 3 fibrosis and in African Americans (83%), warranting some consideration in these specific patient populations [5]. Of note, there is also emerging evidence of SVR rates ≥ 95% with the use of 8 weeks of LDV/SOF therapy in GT4 in non-cirrhotic treatment-naïve patients; however, this is an off-label use [6,7].
Several studies have examined the efficacy of 8 weeks of therapy with GLE/PIB in a variety of patient populations. Noncirrhotic patients with HCV genotype 1, 2, 3, 4, 5, or 6 infection achieved SVR12 rates of at least 95% with 8 weeks of GLE/PIB with SVR12 rates nearly 99% in GT1 infected patients. These rates of SVR12 were similar in subgroup analyses of patients with treatment experience or patients co-infected with HIV across genotypes 1-6 [3].
We have been treating patients with HCV infection for many years through a nurse practitioner-based clinic. This retrospective review will evaluate the outcomes of 8 weeks of DAA therapy in the veteran population at this facility.
To evaluate the achievement of sustained virologic response (SVR) for 8 weeks of therapy with either Ledipasvir/Sofosbuvir (LDV/SOF) or Glecaprevir/Pibrentasvir (GLE/PIB) in our HCV-infected veteran population.
A retrospective chart review of treatment naïve patients with hepatitis C infection who received glecaprevir/pibrentasvir or ledipasvir/sofosbuvir for a planned 8 weeks of therapy in the past four years (January 2015-September 2018) was conducted. Data collected included: age, race, sex, HCV genotype, platelet count, possible presence of cirrhosis (defined by FIB-4 score), relevant co-infections (HIV and hepatitis B), and presence of kidney impairment (CrCl < 30mL/min as calculated by Cockcroft-Gault equation). Treatment outcomes were retrospectively evaluated for each patient through medical record review at baseline visit, 2 weeks of therapy, 4 weeks of therapy, 8 weeks of therapy (final HCV viral load) and at least 12 weeks post-therapy for SVR attainment (non-detectable HCV viral load ≥ 12 weeks after the end of treatment). Treatment failure was defined as a detectable viral load at least 12 weeks after treatment.
Age, platelet count, and liver enzymes (AST/ALT) were used to assess the presence of fibrosis. The FIB-4 score is a non-invasive method to predict the presence of fibrosis (FIB-4 score > 3.25). Sub-group analyses of patients with higher FIB-4 score and of patients age 65 years of age or above were completed.
The project was approved by the Scientific Advisory Committee of our institution as part of the facility’s ongoing performance improvement efforts. The project was not considered research and did not require informed consent. Authors followed privacy laws and data were deidentified after initial validation. Data were analyzed using descriptive statistics in Microsoft Excel.
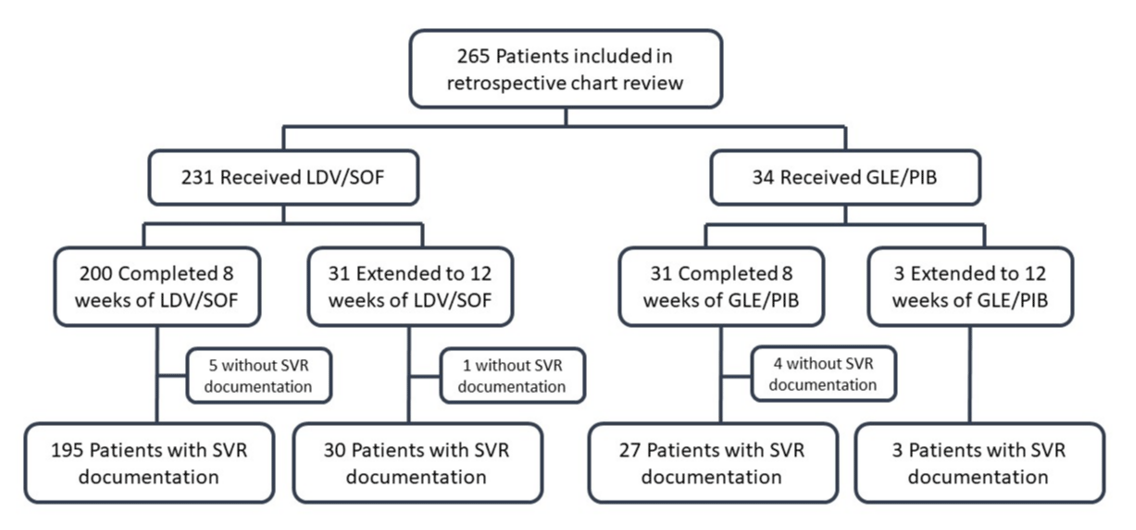
From January 2015-September 2018, a total of 265 treatment naïve veterans were initiated on 8 weeks of therapy with either GLE/PIB or LDV/SOF. Of these, 231 veterans (87%) were initiated on 8 weeks of LDV/SOF and 34 veterans (13%) were initiated on 8 weeks of GLE/PIB. Of the veterans on LDV/SOF, 31 (13%) were switched to 12 weeks of treatment, and of the veterans on GLE/PIB, three (9%) were switched to 12 weeks of treatment, most often due to baseline viral load > 6 million IU/mL (for LDV/SOF), or detectable viral loads at treatment week 4 (Figure 1). The practice of increasing treatment duration is off-labeled and not recommended by treatment guidelines except when baseline viral load is over 6 million with LDV/SOF [4]. The included population was largely Caucasian men (97%) with a mean age of 62 years. Most patients had FIB4 scores below 3.25 (89%), very few had HIV co-infection (2%), none had Hepatitis-B co-infection, and none had substantial kidney impairment (CrCl < 30 mL/min). The majority of HCV genotypes treated were genotype 1a (73%) or 1b (20%) (Table 1).
Figure 1: Flow chart for inclusion criteria. LDV/SOF = ledipasvir/sofobuvir; GLE/PIB = glecaprevir/pibrentasvir.
| Table 1: Baseline Characteristics. | ||||||
| Characteristic | n | (%) | Characteristic | n | (%) | |
| Sex | Genotype | |||||
| Male | 256 | (97%) | 1 | 1 | (0%) | |
| Female | 9 | (3%) | 1a | 193 | (73%) | |
| Age | 1b | 53 | (20%) | |||
| Mean age (range) | 61.6 | (27-76) | 1a/1b | 2 | (1%) | |
| < 65 | 160 | (60%) | 2a | 1 | (0%) | |
| ≥ 65 | 105 | (40%) | 2b | 12 | (5%) | |
| Race | 2a/2c | 1 | (0%) | |||
| White/Caucasian | 166 | (63%) | 3 | 1 | (0%) | |
| Black | 65 | (25%) | 4 | 1 | (0%) | |
| Hispanic/Latino | 8 | (3%) | Cirrhosis | |||
| Other | 26 | (10%) | FIB-4 > 3.25 | 28 | (11%) | |
| HIV Co-infection | FIB-4 ≤ 3.25 | 237 | (89%) | |||
| Yes | 4 | (2%) | ||||
| No | 261 | (98%) | ||||
| HIV: Human Immunodeficiency Virus; Hep-B: Hepatitis B; FIB-4: Fibrosis-4 score. | ||||||
Of the veterans that completed 8 weeks of therapy, the overall SVR rate was 95%. Treatment with GLE/PIB had a higher SVR rate as compared to LDV/SOF (100% vs. 95% respectively). However, the smaller sample size of patients who completed 8 weeks of GLE/PIB (n = 27) vs LDV/SOF (n = 195) may affect the comparability of these SVR rates (Table 2). Of the veterans that completed 12 weeks, (n = 34), we had SVR results for 33 of them, the SVR was also 100% for GLE/PIB (3 of 3) and 97% for LDV/SOV (29 of 30).
| Table 2: 8 Week Treatment (LDV/SOF or GLE/PIB) SVR (n = 231). | ||
| Treatment | Count | SVR Percent* |
| Overall SVR Rate | 212/222 | 95% |
| LDV/SOF | 185 | 95% |
| Failed SVR LDV/SOF | 10 | 5% |
| GLE/PIB | 27 | 100% |
| Failed SVR GLE/PIB | 0 | 0% |
| SVR Not Available (n/a) | 9 | -- |
| *excluding SVR values n/a | ||
| GLE/PIB: Glecaprevir/Pibrentasvir; LDV/SOF: Ledipasvir/Sofosbuvir; SVR: Sustained Virologic Response |
||
In a sub-group analysis of patients with FIB-4 scores > 3.25 (median score 4.31, range score 3.28-9.21), we found that patients overall had 100% SVR attainment. However, these patients were more likely to be extended to 12-week treatment durations than our overall population (Table 3), and in another sub-group analysis of elderly patients defined as 65 years of age or older, we found similar overall SVR rates for 8 weeks of therapy as compared to those less than 65 years of age (94% vs. 96% respectively) (Table 4).
| Table 3: SVR rate for patients with FIB-4 > 3.25. | ||
| Treatment | Count | SVR Percent* |
| FIB-4 > 3.25, 8 weeks tx | 20 | -- |
| FIB-4 > 3.25, 12 weeks tx | 8 | -- |
| SVR for FIB-4 > 3.25, Overall | 27 | 100% |
| SVR for FIB-4 > 3.25, 8 weeks tx | 19 | 100% |
| SVR for FIB-4 > 3.25, 12 weeks tx | 8 | 100% |
| SVR Not Available (n/a) | 1 | -- |
| *excluding SVR values n/a | ||
| FIB-4: Fibrosis-4 score; SVR: Sustained Virologic Response | ||
| Table 4: SVR for patients ≥ 65 years of age versus 65 years receiving 8 weeks. | ||
| Elderly | Count | SVR Percent* |
| ≥ 65 years old, Overall SVR | 83 | 94% |
| GLE/PIB | 13/13 | 100% |
| LDV/SOF | 60/65 | 92% |
| SVR not available (n/a) | 5 | ---- |
| < 65 years old, Overall SVR | 139 | 96% |
| GLE/PIB | 14/14 | 100% |
| LDV/SOF | 114/119 | 96% |
| SVR not available (n/a) | 4 | ---- |
| *excluding SVR values n/a | ||
| GLE/PIB: Glecaprevir/Pibrentasvir; LDV/SOF: Ledipasvir/Sofosbuvir; SVR: Sustained Virologic Response | ||
Overall, our analysis shows that SVR rates with 8 weeks of DAA treatment in our veteran population are similar to those reported in previous literature. SVR rate with 8 weeks of therapy with LDV/SOF was approximately 95% which is comparable to that reported in the ION-3 trial that garnered the approval for the 8 weeks regimen. In the ION-3 trial, included 647 treatment naïve non-cirrhotic subjects and resulted in 94% SVR rate overall after 8 weeks of LDV/SOF treatment and 97% SVR rate for those with a baseline HCV RNA < 6 million IU/mL [8]. In regard to treatment SVR rates with GLE/PIB, our rates were also similar to those reported in previous literature. Noncirrhotic patients with HCV genotype 1, 2, 3, 4, 5, or 6 infection achieved SVR12 rates of at least 95% with 8 weeks of GLE/PIB with SVR12 rates nearly 99% in genotype 1 infected patients [3].
Although approximately 13% of our patients were extended to 12 weeks of DAA treatment, the reason for extension was most often because of elevated baseline viral load or a detectable viral load at 4 weeks of treatment. Especially for LDV/SOF, as mentioned previously, it is established that SVR rates are not as high in patients with elevated baseline viral loads, and therefore, extending the treatment duration follows guideline recommendations [4].
Another sub-population of concern for efficacy of 8 weeks DAA treatment options is patients with cirrhosis. Treatment with 8 weeks of LDV/SOF had lower SVR12 rates (93%) in patients with high FIB4 scores. And for GLE/PIB, literature shows a similar trend with decreased SVR rates in patients with cirrhosis [9]. In the EXPEDITION trial the SVR12 rate was 100% (n = 136/136; 95% CI, 97.3%–100%) in patients without cirrhosis treated for 8 weeks and 93% (n = 14/15; 95% CI, 70.2%–98.8%) in patients with compensated cirrhosis treated for 12 weeks [10]. This led to the guidelines recommending 8 weeks of therapy only in patients without cirrhosis [4]. However, another study showed comparable SVR rates on 8-16 weeks of GLE/PIB treatment for patient with and without cirrhosis based on liver biopsy, transient elastography, or screening results (96.4% vs 97.5%) [11].
Interestingly, in our sub-group analysis, patients with FIB-4 score > 3.25 (positive predictive value of 65% for advanced fibrosis with a specificity of 97%) [12], did not have a diminished SVR response in either LDV/SOF or GLE/PIB group. All patients had 100% SVR rate. However, a limitation in drawing comparison of our results with those reported in previous literature is that we only used the FIB-4 score to predict cirrhosis and some patients received the longer 12-week regimen. Confirmation of diagnosis of cirrhosis for patients with FIB-4 scores > 3.25 was not evaluated in this population; therefore, generalizability of these findings is limited.
A useful finding of this project is the added evidence for 8 weeks DAA treatment efficacy in an elderly population which has not been reported elsewhere. Forty percent of our patients with SVR results were elderly (age ≥ 65 years) and we noted similar SVR attainment as compared to the younger population. A recent analysis of previous large trials with GLE/PIB reviewed efficacy specifically in elderly. The authors found that SVR rates with GLE/PIB were similar for patients aged ≥65 years and patients aged < 65 years (SVR12 rates of 97.9% and 97.3% respectively). The rates were not significantly different between the two age groups [13]. Of note, only 29% of the patients aged ≥65 years were on 8 weeks of DAA treatment as compared to 37% of patients aged <65 years. SVR rates were not reported based on treatment duration.
A main limitation of our report is that it was a single center retrospective analysis which will limit the generalizability of our findings. We also excluded patients without final viral loads (4%) to calculate SVR rates, which may have affected the results although it is expected to result in a negligible change in overall SVR rate.
Treatment for 8 weeks of DAA therapy in the veteran population at this facility showed an overall SVR rate of 95% which is similar to those reported in the literature. However, the small population size on GLE/PIB may affect the generalizability of our results to other populations.
- Centers for Disease Control and Prevention. Viral Hepatitis: Hepatitis C Information. 2019.
- Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2014; 61: 77-87. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25069599
- Abutaleb A, Kottilil S, Wilson E. Glecaprevir/pibrentasvir expands reach while reducing cost and duration of hepatitis C virus therapy. Hepatol Int. 2018; 12: 214-222. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29845496
- American Association for the Study of Liver Diseases, and Infectious Diseases Society of America. HCV guidance: recommendations for testing, managing, and treating hepatitis C. 2019
- Andres J, Lott S, Qureshi K. Eight-Week Outcomes of Ledipasvir/Sofosbuvir in Noncirrhotic Treatment-Naive Patients with Hepatitis C: Analysis of Pharmacy-Based Data. J Manag Care Spec Pharm. 2018; 24: 23-28. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29290174
- Babatin MA, AlGhamdi AS, Assiri AM, AlBiladi H, AlOthmani HS, et al. Treatment efficacy of ledipasvir/sofosbuvir for 8 weeks in non-cirrhotic chronic hepatitis C genotype 4 patients. Saudi J Gastroenterol. 2019; 25: 55-60. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30117490
- Shiha G, Esmat G, Hassany M, Soliman R, Elbasiony M, et al. Ledipasvir/sofosbuvir with or without ribavirin for 8 or 12 weeks for the treatment of HCV genotype 4 infection: Results from a randomised phase III study in Egypt. Gut. 2019; 68: 721-728. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29666174
- Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014; 370: 1879-1888. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24720702
- Wyles D, Poordad F, Wang S, Alric L, Felizarta F, et al. (2018) Glecaprevir/pibrentasvir for hepatitis C virus genotype 3 patients with cirrhosis and/or prior treatment experience: A partially randomized phase 3 clinical trial. Hepatology. 2018; 67: 514-523. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28926120
- Rockstroh JK, Lacombe K, Viani RM, Orkin C, Wyles D, et al. Efficacy and Safety of Glecaprevir/Pibrentasvir in Patients Coinfected With Hepatitis C Virus and Human Immunodeficiency Virus Type 1: The EXPEDITION-2 Study. Clin Infect Dis. 2018; 67: 1010-1017. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29566246
- Gane E, Poordad F, Zadeikis N, Valdes J, Lin CW, et al. Safety and Pharmacokinetics of Glecaprevir/Pibrentasvir in Adults with Chronic Genotype 1-6 Hepatitis C Virus Infections and Compensated Liver Disease. Clin Infect Dis. 2019; 69: 1657-1664. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30923816
- Tavabie OD. Should we screen for cirrhosis? BMJ. 2017; 358: j3233.
- Foster GR, Asselah T, Kopecky-Bromberg S, Lei Y, et al. Safety and efficacy of glecaprevir/pibrentasvir for the treatment of chronic hepatitis C in patients aged 65 years or older. PLoS One. 2019; 14: e0208506. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30601818